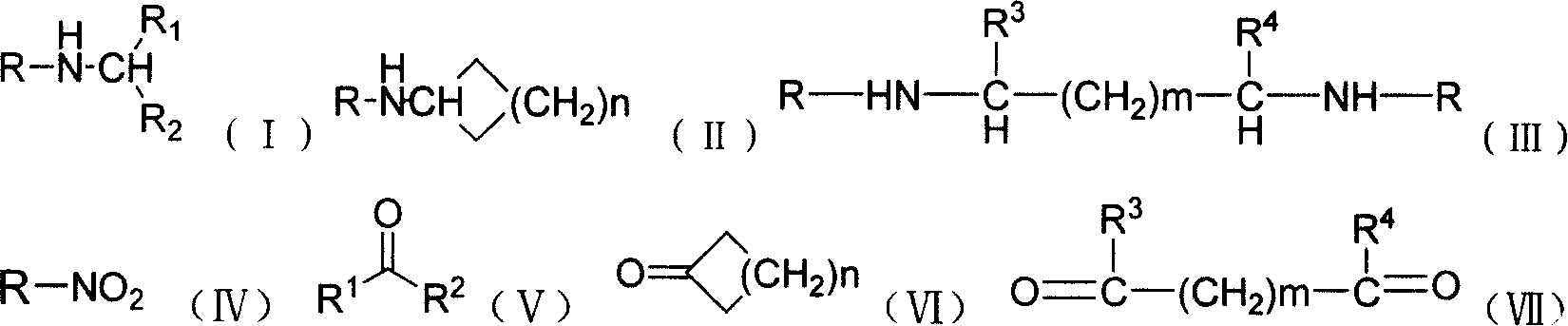
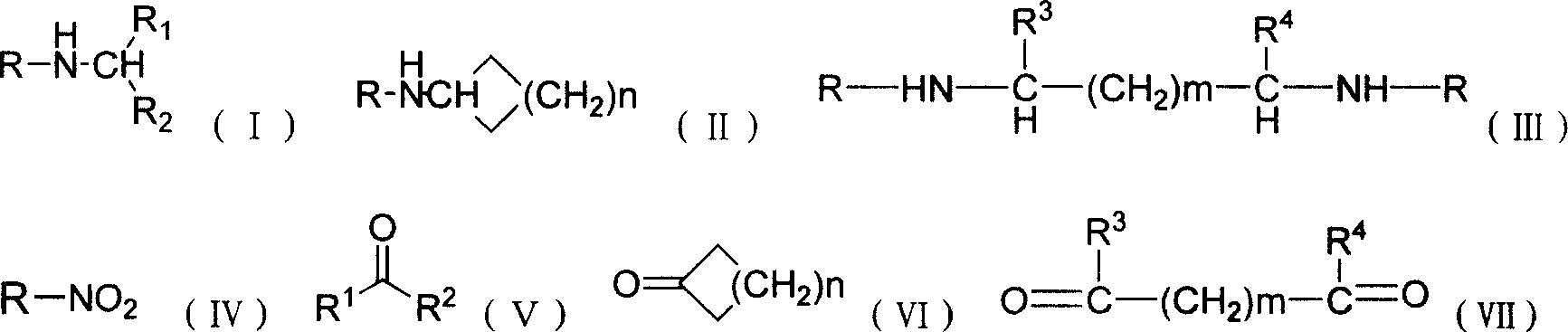
Production of secondary-amine compound
A technology of ketone compounds and compounds, which is applied in the field of preparation of secondary amine compounds, can solve the problems of long reaction steps in preparation methods, different selectivity of preparation methods, product-mixture, etc., and achieve low production cost, broad application prospects, and excellent reaction process simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: catalyst preparation
[0027] Raney nickel (Raney Ni) catalyst preparation:
[0028] In a 1L three-necked flask equipped with stirring, add 500ml of 20% sodium hydroxide aqueous solution, heat to 90°C, and under stirring, add 40g of nickel-aluminum alloy (Ni:Al=50:50, Hangzhou Jia Lee Metal Co., Ltd.). The speed of addition should keep the temperature of the solution between 90 and 95°C, and the addition should be completed within about 30 minutes. Stirring was continued for 1 hour. Let stand to allow the nickel to settle, and pour off the supernatant. Wash 5 times by pouring method, each time with 200ml distilled water. Then wash 5 times with ethanol pouring method, 50ml each time. The prepared Raney Ni catalyst was stored in absolute ethanol for subsequent use.
[0029] Preparation of supported nickel (Ni) catalyst:
[0030]In a 150mL three-necked flask equipped with stirring, add 6.22g of nickel nitrate and 30mL of water, add 20g of silica gel af...
Embodiment 2
[0036] In a volume of 500 milliliters, in an autoclave equipped with stirrer, electric heater, gas guide pipe, pressure and temperature indicating instrument, 0.93 gram of glyoxal, 4.0 gram of nitrobenzene, 0.3 gram of embodiment 1 are loaded into Raney Ni catalyst and 100 ml ethanol. Then, the reaction system was successively replaced three times with nitrogen and hydrogen respectively, and the temperature was controlled at about 80°C. 2 Catalytic hydrogenation under pressure until hydrogen absorption is complete. After cooling, the material was discharged, filtered, and the solvent was evaporated under reduced pressure to obtain 4.8 grams of N,N'-diphenylethylenediamine product. The product contained 99% of N,N'-diphenylethylenediamine through high performance liquid chromatography analysis. The yield was 97%.
Embodiment 3
[0038] In a volume of 500 milliliters, in an autoclave equipped with stirrer, electric heater, air duct, pressure and temperature indicating instrument, charge 2.9 grams of 2,3-dichlorobenzaldehyde, 2.6 grams of nitrobenzene, 0.5 grams Raney Ni catalyst prepared in Example 1 and 100 milliliters of ethanol. Then, the reaction system was successively replaced three times with nitrogen and hydrogen respectively, and the temperature was controlled at about 80°C. 2 Catalytic hydrogenation under pressure until hydrogen absorption is complete. After cooling, the material is discharged, filtered, and the solvent is evaporated under reduced pressure to obtain 5.4 grams of N-(2,3-dichlorophenyl)aniline product. The product contains N-(2,3-dichlorophenyl) through high performance liquid chromatography analysis. Aniline 97.5%, the yield is 94.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com