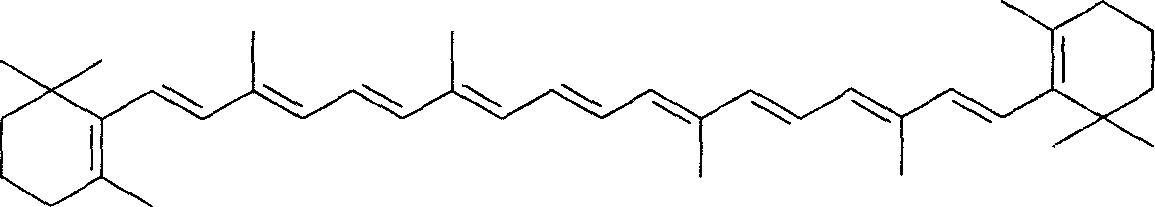
Method for preparing full-trans beta-carrotin
A carotene and all-trans technology, applied in the direction of organic chemistry, can solve the problems of large solvent consumption and low transposition yield, and achieve the effect of less solvent consumption, high reaction yield and large processing capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] In a 1000ml three-necked flask, add 100g of Dunaliella β-carotene extract containing 48% cis-isomer and 1000g of glycerol, control the reaction temperature to be 130°C, and react in the dark for 3 hours under nitrogen protection, then cool to 70°C °C, the reaction was continued for 12 hours. After the reaction, the mixture was filtered while hot, washed with water, and dried to obtain 98 g of β-carotene containing 99% all-trans isomer.
Embodiment 2
[0018] Add 500g of synthetic β-carotene containing 67% cis-isomer and 500g of propylene glycol in a 1000ml three-necked flask, control the reaction temperature to 160°C, react under nitrogen protection and avoid light for 5 hours, then cool down to 100°C, and continue the reaction for 25 Hour. After the reaction, the mixture was filtered while hot, washed with water, and dried to obtain 492 g of β-carotene containing 98% all-trans isomer.
Embodiment 3
[0020] In a 1000ml three-necked flask, add 200g of synthetic β-carotene containing 26% cis-isomer and 800g of glycerol monoacetate, control the reaction temperature to be 120°C, react in the dark for 6 hours under nitrogen protection, then cool down to 80°C, The reaction was continued for 22 hours. After the reaction, the mixed solution was filtered while hot, washed with water and dried to obtain 195 g of β-carotene containing 98% all-trans isomer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com