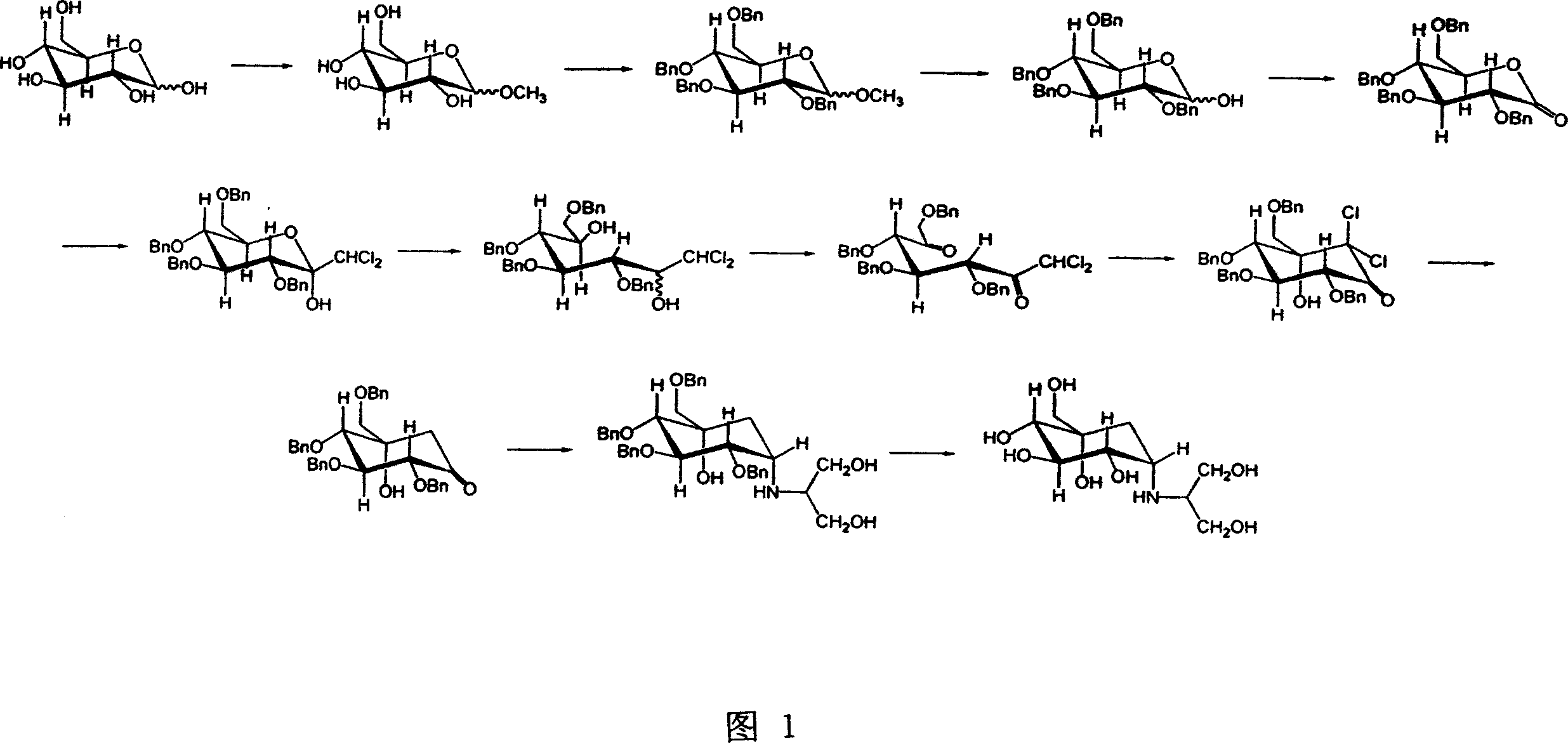
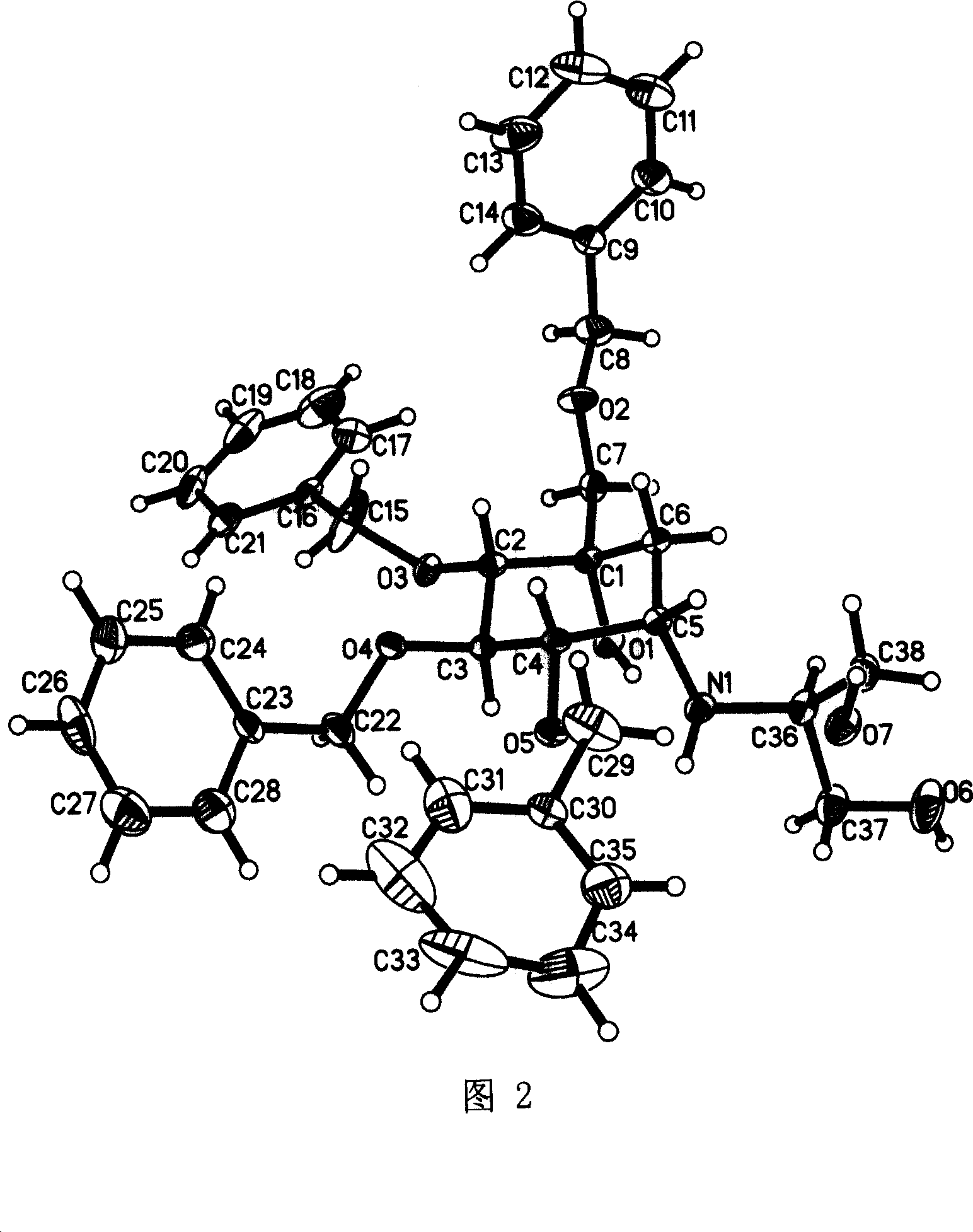
Tetrabenzyl voglibose crystallizing and preparing process
A technology of benzyl and benzyloxymethyl, which is applied in the field of crystallization of tetrabenzyl voglibose, can solve the problems of difficult removal of impurities, cumbersome separation process of Jinggang mycylamine, a large amount of labor and energy consumption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Oily (1S)-(1(hydroxyl),2,4,5 / 1,3)-2,3,4-tri-oxo-benzyl-5-[(2-hydroxyl-1-( Hydroxymethyl) ethyl) amino]-1-carbon-benzyloxymethyl-1,2,3,4-cyclohexanetetraol (abbreviated as tetrabenzyl voglibose (the same below), (II )) (with reference to the literature J.Org.Chem.1992,57,3651 method)
[0049](1S)-(1(hydroxyl),2,4,5 / 1,3)-2,3,4-tri-oxo-benzyl-1-carbon-benzyloxymethyl-5-oxo-1 , 2,3,4-cyclohexanetetraol (6.0g, 10.8mmol) and 2-amino-1,3-propanediol (3.0g, 33mmol) were dissolved in 30ml of methanol, and cyanoboron was added in portions at room temperature Sodium hydride (1.5g, 24mmol), after adding, continue to stir at room temperature for 16 hours, concentrate the reaction solution, dissolve the residue with 300ml of ethyl acetate, wash with 100ml of water, and wash twice with 100ml of 1% aqueous hydrochloric acid solution, 5% Wash twice with 100 ml of aqueous sodium carbonate solution, twice with 100 ml of saturated brine, and dry over anhydrous sodium sulfate. ...
Embodiment 2
[0050] Example 2 Preparation of tetrabenzyl voglibose (II) crystals
[0051] Dissolve 1.0 g of oily tetrabenzyl voglibose in 2.5 ml of ethyl acetate, add 6 ml of cyclohexane under stirring, and stir the solution at room temperature for 1.5 hours to form colorless granular crystals, continue to Stand for 5 hours, then place at 0-5°C for 5 hours, filter, and dry the crystals in vacuum at room temperature for 10 hours to obtain 0.76 g of colorless granular crystals. Content determined by HPLC method: 98.5%; mp88.2-90.8°C; [α] 22 D +30.8°(cl, chloroform); 1 H NMR (CDCl 3 , 500Hz), δ: 1.63 (1H, dd, J = 2.8, 15.1Hz), 1.91 (1H, dd, J = 2.9, 15.1Hz), 2.78 (1H, m), 3.19 (1H, d, J = 8.6 Hz), 3.39(1H, m), 3.54(1H, d, J=8.6Hz), 3.62-3.73(6H, m), 4.13(1H, t, J=9.6Hz), 4.39(2H, s), 4.59 (1H, d, J = 11.1), 4.64 (1H, d, J = 11.4), 4.72 (1H, d, J = 11.4), 4.82 (1H, d, J = 10.6), 4.91 (1H, d, J=11.2), 4.93 (1H, d, J=10.7), 7.24-7.35 (20H, m)
Embodiment 3
[0052] Example 3 Preparation of tetrabenzyl voglibose (II) crystals
[0053] Dissolve 3.0 g of oily tetrabenzyl voglibose in 10 ml of isopropyl ether, add 25 ml of n-hexane while stirring, and stir the solution at room temperature for 1 hour to form colorless granular crystals, and continue to stand at room temperature for 1 hour. hours, and then placed at 0-5°C for 3 hours, filtered, and the crystals were vacuum-dried at room temperature for 12 hours to obtain 2.5 g of colorless granular crystals. Content determined by HPLC method: 98.7%; mp88.5-90.7°C; [α]22D+30.6° (cl, chloroform), and the hydrogen spectrum data are consistent with those in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com