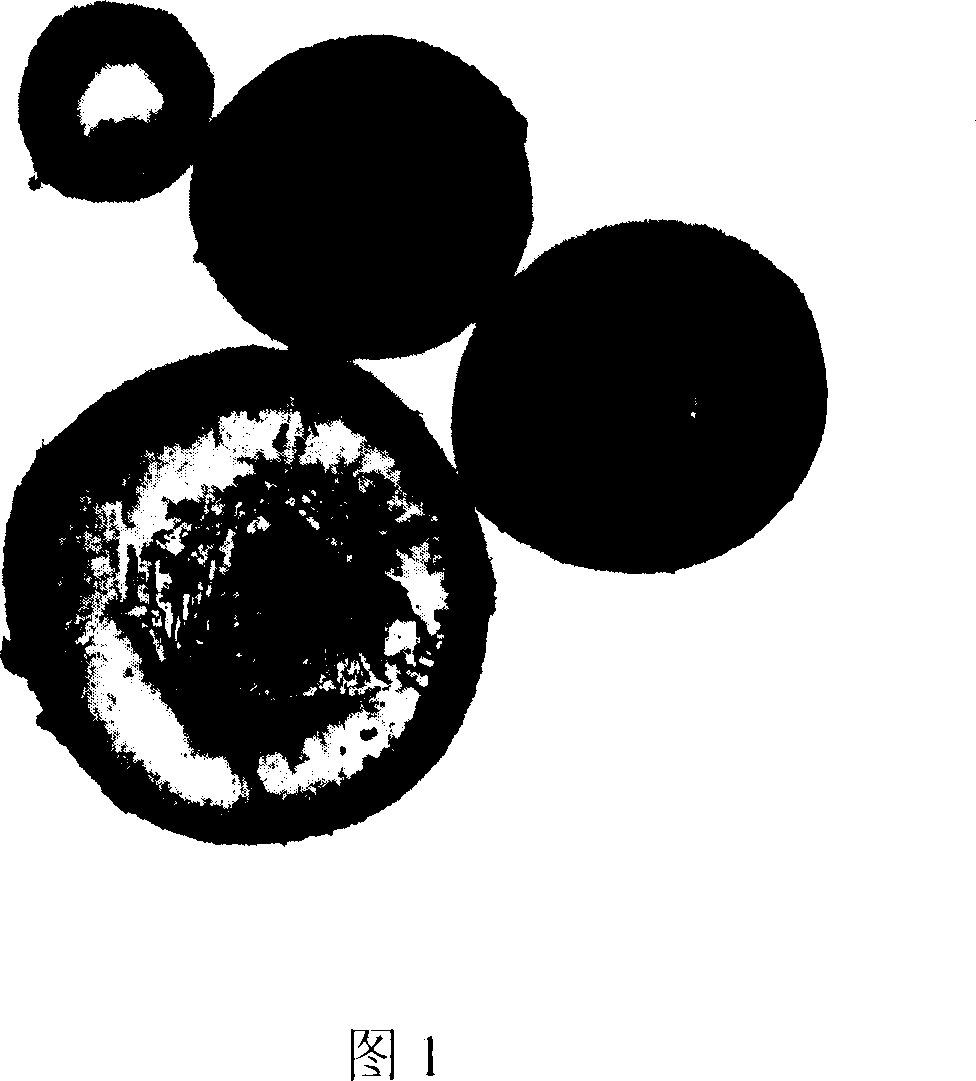Glycopeptide conjugate microsphere or microcapsule and its prepn process
A conjugate and glycopeptide technology, applied in the field of glycopeptide conjugate microspheres or microcapsules and their preparation through interfacial polymerization, can solve the problems of complex microcapsule steps and achieve good tissue compatibility and degradability properties, simple preparation process, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Dissolve 4 grams of chitosan in 100ml of deionized water (containing 2.8ml of glacial acetic acid), add 100ml of absolute ethanol and 32ml of pyridine after all dissolve, stir until clear, then add 3.6ml of acetic anhydride, and stir at room temperature for 2 hours. The reaction solution was poured into ethanol and filtered with suction to obtain a white precipitate. The crude product was dissolved in water, and the insoluble matter was removed by centrifugation. The filtrate was added with 200ml of absolute ethanol, and the supernatant was discarded after centrifugation. The precipitate was washed with acetone and anhydrous ether in turn, and dried in vacuum at room temperature for 24 hours to obtain a white flocculent solid. The degree of deacetylation of the product measured by acid-base titration was 43%.
[0047] Under Ar protection, prepare 0.1g / 10ml above-mentioned chitosan aqueous solution in 100ml three-neck flask. Weigh 0.6825g L-leucine-N-carboxylic acid int...
Embodiment 2
[0049] Dissolve 4 grams of chitosan in 100ml of deionized water (containing 2.8ml of glacial acetic acid), add 100ml of absolute ethanol and 32ml of pyridine in turn after all the dissolution, stir until clear, then add 3.6ml of acetic anhydride, and stir at room temperature for 3 hours. The reaction solution was poured into ethanol and filtered with suction to obtain a white precipitate. Dissolve the crude product with an appropriate amount of deionized water, centrifuge to remove insoluble matter, add 200ml of absolute ethanol to the filtrate, discard the supernatant after centrifugation, wash the precipitate with acetone and anhydrous ether in turn, and dry it in vacuum at room temperature for 24 hours to obtain a white flocculent solid. The degree of deacetylation of the product measured by acid-base titration was 43%.
[0050] Under Ar protection, prepare 0.1g / 10ml above-mentioned chitosan aqueous solution in 100ml three-neck flask. Weigh 0.4263g L-leucine-N-carboxylic ...
Embodiment 3
[0053] Dissolve 4 grams of chitosan in 100ml of deionized water (containing 2.8ml of glacial acetic acid). After all of the solution is dissolved, add 100ml of absolute ethanol and 32ml of pyridine in turn, stir until clear, then add 3.4ml of acetic anhydride, and stir at room temperature for 4 hours. The reaction solution was poured into ethanol and filtered with suction to obtain a white precipitate. Dissolve the crude product with an appropriate amount of deionized water, centrifuge to remove insoluble matter, add 200ml of absolute ethanol to the filtrate, discard the supernatant after centrifugation, wash the precipitate with acetone and anhydrous ether in turn, and dry it in vacuum at room temperature for 24 hours to obtain a white flocculent solid. The degree of deacetylation of the product measured by acid-base titration was 50%.
[0054] Under Ar protection, prepare 0.1g / 10ml above-mentioned chitosan aqueous solution in 100ml three-neck flask. Weigh 1.3382g N ε -Ben...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com