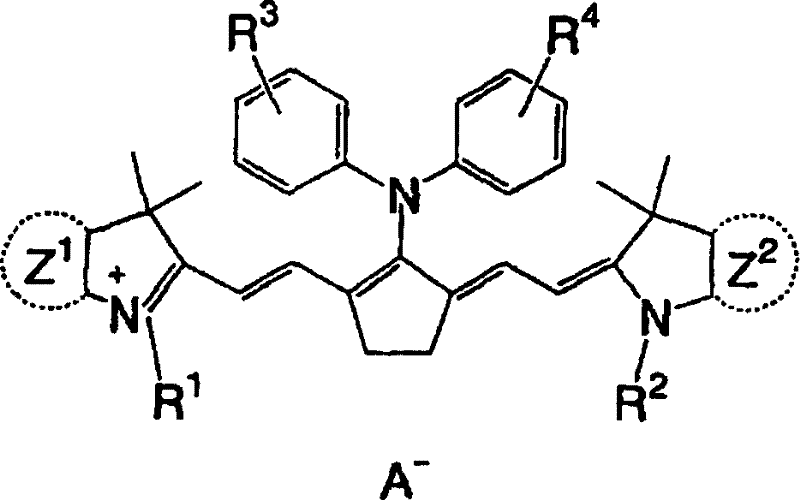
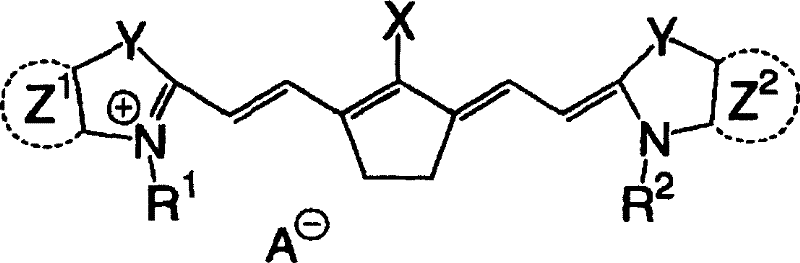
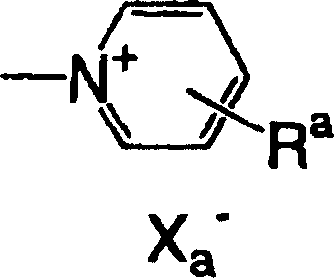
Lithographic printing plate precursor, lithographic printing method, and novel cyanine dye
一种平版印刷版、花青染料的技术,应用在平版印刷版前体,新型花青染料领域,能够解决印刷材料负面影响等问题,达到防止浮渣、机上显影性能优异、防止湿润水着色的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0231] The preparation methods of microcapsules include, for example, methods using coacervation described in U.S. Patent Nos. 2,800,457 and 2,800,458, methods using interfacial polymerization described in U.S. Patent No. 3,287,154, JP-B-38-19574 and JP-B-42-446, U.S. Methods using deposition of polymers described in patents 3,418,250 and 3,660,304, methods using isocyanate polyol wall materials described in US Patent 3,796,669, methods using isocyanate wall materials described in US Patent 3,914,511, US Patents 4,001,140, 4,087,376, and 4,089,802 The method described in using urea-formaldehyde-type or urea-formaldehyde-resorcinol-type wall-forming materials, using wall materials, such as the method of melamine-formaldehyde resin or hydroxycellulose described in U.S. Patent 4,025,445, by The in situ process of monomer polymerization described in JP-B-36-9163 and JP-B-51-9079, the spray drying method described in British Patent 930,422 and US Patent 3,111,407, and the method d...
Embodiment 1
[0327] Synthesis Example 1: Synthesis of Specific Cyanine Dye (Compound (IR-32)) and (Compound (IR-26))
[0328] A mixture of 37.9 g of 3-methoxypropanol and 53.9 g of p-toluenesulfonyl chloride in 50.2 g of pyridine was stirred for 3 hours while maintaining a reaction temperature of 0-10° C., then extracted with ethyl acetate to obtain 91.2 g (89% yield) of 3-methoxypropyl tosylate.
[0329] A mixture of 55 g of 3-methoxypropyl tosylate thus obtained and 39.0 g of 2,3,3,5-tetramethyl-3-H-indole was stirred at 120° C. for 3 hours, followed by Cool to room temperature. Then, 47.7 g of 2,5-bis[(phenylamino)methylene]cyclopentylene diphenylammonium tetrafluoroboric acid, 23.0 g of acetic anhydride, 56.9 g of triethylamine and 220 ml of 2-propanol, and the mixture was stirred at 80°C for 3 hours. After the reaction was completed, the mixture was cooled to room temperature, and 90 ml of water was added thereto. Crystals thus deposited were collected by filtration and washed wel...
Embodiment 2
[0344] Synthesis Example 2: Synthesis of Specific Cyanine Dye (Compound (IR-2))
[0345] A mixture of 2-[2-(2-methoxyethoxy)ethoxy]ethyl toluenesulfonate and 79g of 2,3,3,5-tetramethyl-3-H-indole Stir at 120°C for 3 hours, then cool to room temperature. Then, 120.4 g of 2,5-bis[(phenylamino)methylene]cyclopentylenediphenylammonium tetrafluoroboric acid, 46.5 g of acetic anhydride, 115.1 g of triethylamine, and 1.1 liters of tetrafluoroboric acid were added thereto. 2-propanol, and the mixture was stirred at 80 °C for 3 hours. After the reaction was completed, the mixture was cooled to room temperature and 1.5 L of DMAc was added thereto. Add the reaction solution dropwise to the KPF containing 2.2kg 6 , 6 liters of DMAc and 24 liters of water in a mixed solution. Then, the crystals thus deposited were collected by filtration and washed sufficiently with water to obtain 195.7 g (yield 85%) of a specific cyanine dye (IR-2).
[0346] For the specific cyanine dye (IR-2) thus ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com