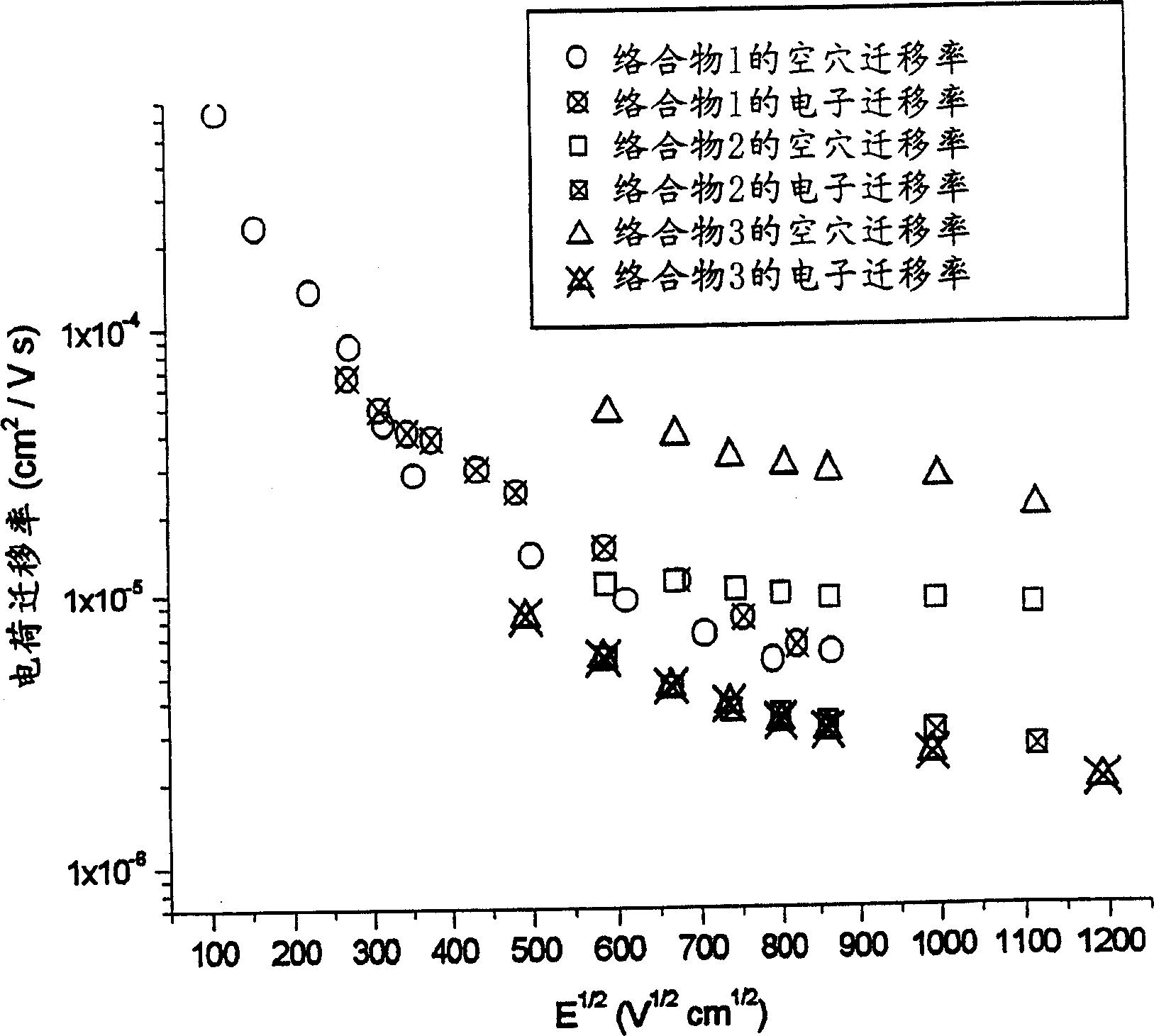
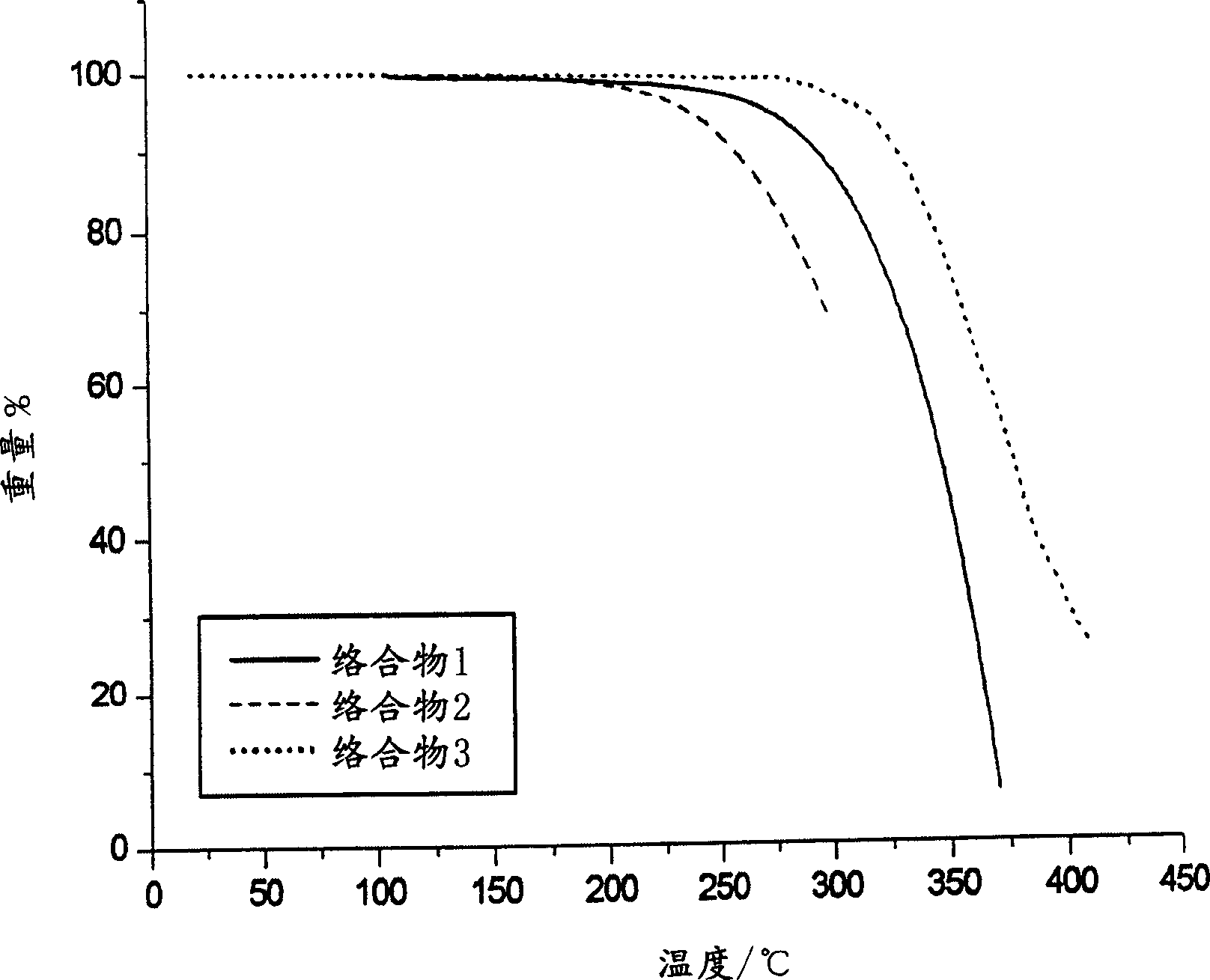
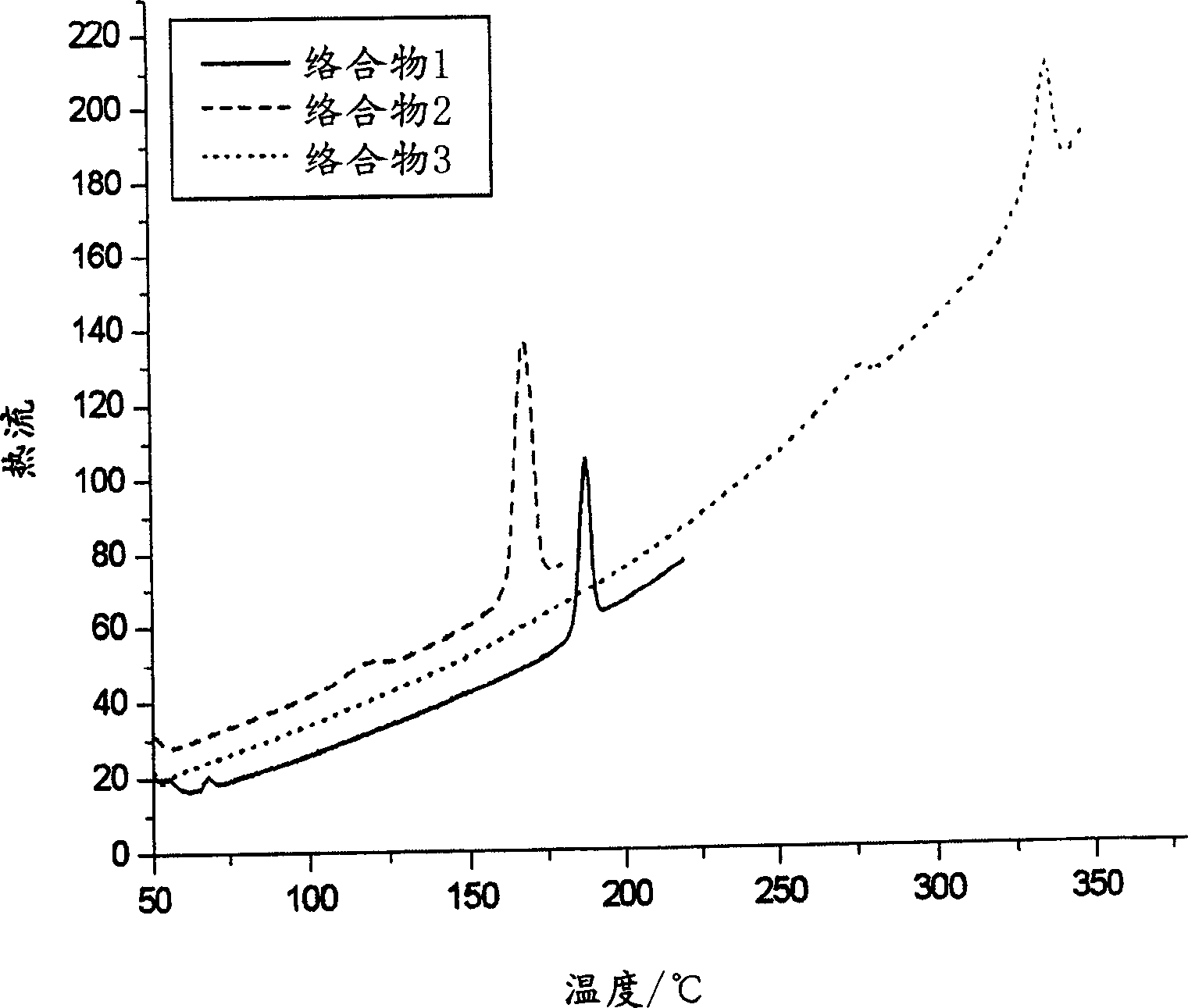
Materials for electroluminescent devices
A technique of electroluminescence and emission layer, applied in the field of efficiently preparing these materials, materials containing borazane
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] The preparation of embodiment 1-compound 2
[0115] Freshly distilled aniline (5 g, 0.055 mol) was introduced under argon into a round bottom flask containing borane triethylamine compound (9 mL, 0.061 mol). The mixture was heated to 80°C for a total of 16 hours and then further heated to 180°C. Excess reactants were then removed under vacuum. The reaction mixture was cooled to room temperature to collect the product, which was then purified by high vacuum sublimation. A white solid was obtained (3 g, 53% yield).
[0116] 1 H NMR (300MHz, CDCl 3 )δ7.35 (t, J=6.90Hz, 6H), 7.25-7.18 (m, 9H), 5.00 (bs, 3H) (Note: t=triplet, m=multiplet, b=broad, s= Unimodal). 13 C NMR (75MHz, CDCl 3 ,) δ148.0, 128.8, 125.2, 124.7. FAB-MS (m / z): 309 [M + ]. Melting point 167°C (DSC). T g : 121°C. T d : 203°C.
Embodiment 2
[0117] The preparation of embodiment 2-compound 3
[0118] Freshly distilled acetonitrile (2 g, 0.048 mol) was introduced under argon into the round bottom flask containing calcium chloride dried chlorobenzene. Bubbles of boron trichloride were then continuously bubbled through the solution until smoke was observed on the neck of the bottle, indicating that all the acetonitrile had been consumed. Dry powdered ammonium chloride (2.8 g) was added and the reaction mixture was heated at reflux for 3 hours. The reaction mixture was cooled to room temperature and the chlorobenzene was removed by cannula. Next, 150 ml of benzene was introduced into the reaction mass, and then a solution of 15 g (0.088 mol) of diphenylamine in 100 ml of benzene was added dropwise to the reaction mixture. The reaction mixture was refluxed for an additional 16 hours. The white product (4.5 g, 48% yield) was collected by filtration and purified by high vacuum sublimation. FAB-MS (m / z): 582[M + ]. E...
Embodiment 3
[0121] * = not measured
[0122] n.o. = not observed
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com