Metal compound, material for forming thin film and method for preparing thin film
一种金属化合物、制造方法的技术,应用在薄膜的制造领域,能够解决不具有挥发性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
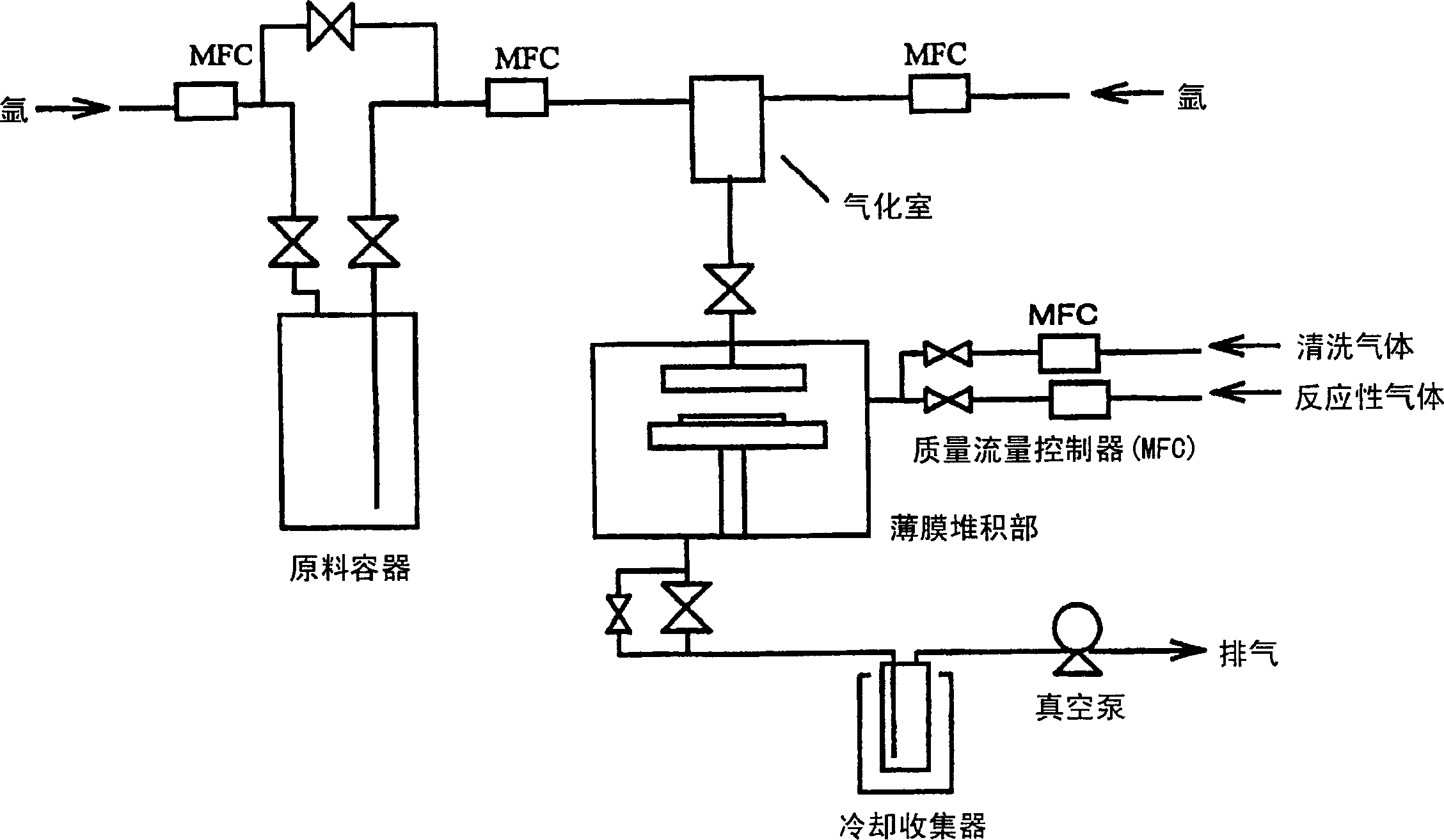
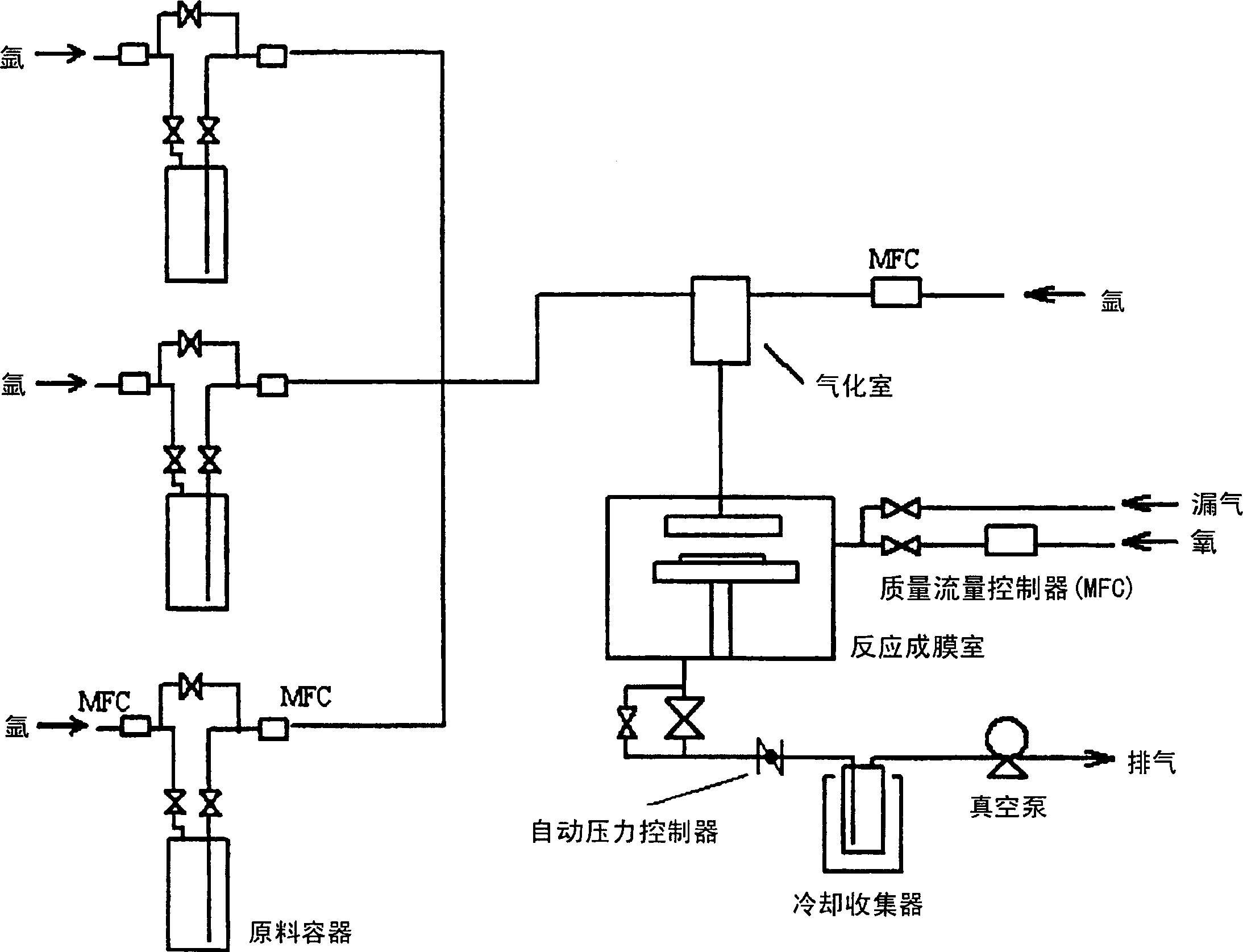
Image
Examples
Embodiment 1
[0050] [Example 1] Production of Compound No.1
[0051] Under a dry argon atmosphere, add 0.478 mol of lead dichloride, 2000 ml of dehydrated diethyl ether, 0.935 mol of bis(trimethylsilyl)amide lithium in a light-shielding reaction flask, and stir at 25°C for 24 Hour. Diethyl ether was distilled off as a solvent, 3000 ml of dehydrated hexane was added to the obtained residue, and the solid phase was filtered to obtain a solution. 1.03 mol of 1-dimethylamino-2-methyl-2-propanol was added dropwise to this solution, and after stirring at 25°C for 24 hours, hexane and by-product hexamethyldisilazide were distilled off. alkyl. White crystals were obtained at a yield of 38% from fractions at 13 to 15 Pa and a tower top temperature of 90 to 95°C. The white crystals were further purified by distillation under reduced pressure to obtain crystals. The recovery rate of this purification was 90%. The obtained crystal was identified as the target compound No.1. The analytical values...
Embodiment 2
[0058] [Example 2] Preparation of Compound No.13
[0059] Under a dry argon atmosphere, drop 0.100 mol of tetrakis(2-propoxy)titanium, 60 ml of dehydrated xylene, 0.440 mol of 1-dimethylamino-2-methyl-2 into the reaction flask -propanol, by-product 2-propanol was distilled off while reacting at 135°C for 18 hours. Xylene was distilled off, and the obtained residue was distilled off under reduced pressure. A light yellow liquid was obtained at a yield of 48% from the fraction at 5-7 Pa and a tower top temperature of 122-124°C. The light yellow liquid was further purified by distillation under reduced pressure to obtain a liquid. The recovery rate of this purification was 94%. The resulting liquid was identified as the target compound No.13. The analytical values of the obtained liquid are shown below.
[0060] (analytical value)
[0061] (1) Elemental analysis (metal analysis: ICP-AES)
[0062] Titanium: 9.43% by mass (theoretical value: 9.34%)
[0063] (2) 1 H-NMR (...
Embodiment 3
[0066] [Example 3] Preparation of Compound No.25
[0067] Under a dry argon atmosphere, 0.100 moles of tetrakis(2-propoxy) zirconium 2-propanol adduct, 60 ml of dehydrated xylene, 0.440 moles of 1-dimethyl Amino-2-methyl-2-propanol, while distilling off the by-product 2-propanol, reacted at 135°C for 10 hours. Xylene was distilled off, and the obtained residue was distilled off under reduced pressure. A colorless liquid was obtained at a yield of 53% from the fraction at 8 to 10 Pa and a tower top temperature of 139 to 140°C. This colorless liquid was further purified by distillation under reduced pressure to obtain a liquid. The recovery rate of this purification was 92%. The resulting liquid was identified as the target compound No. 25. The analytical values of the obtained liquid are shown below.
[0068] (analytical value)
[0069] (1) Elemental analysis (metal analysis: ICP-AES)
[0070] Zirconium: 16.8% by mass (theoretical value is 16.4%)
[0071] (2) 1 H-NMR...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com