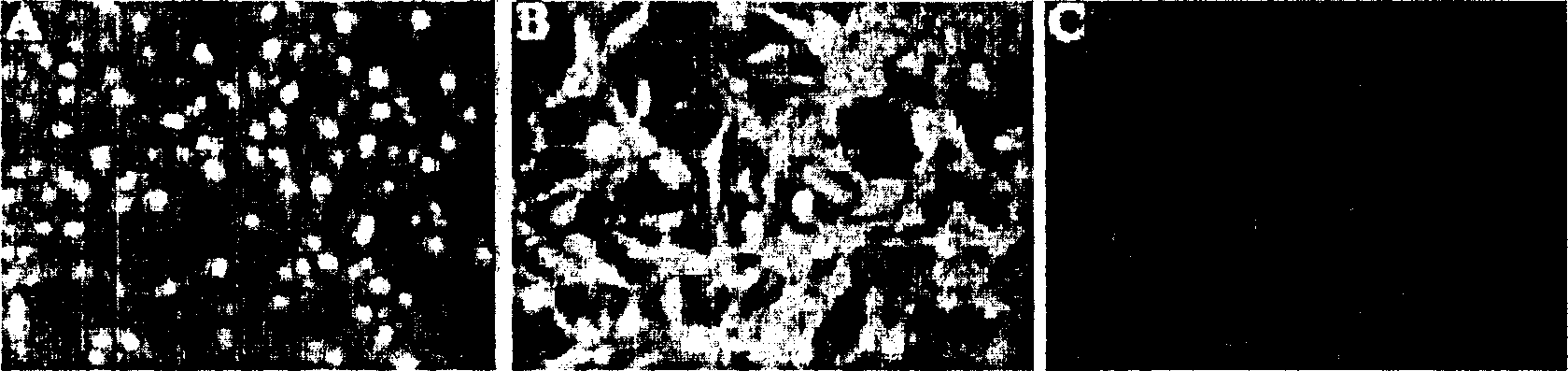Method of preparing cell for transplantation
A cell and original cell technology, applied in biochemical equipment and methods, animal cells, vertebrate cells, etc., can solve the problem of not being able to induce differentiation significantly, and achieve the effect of high cell survival rate and high cell incorporation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: Method of inducing cardiomyocytes from bone marrow stem cells by treatment with biological growth factors
[0068] More than four weeks after myocardial infarction, 15-20 ml of bone marrow stem cells were obtained from dog ilium using a heparin-soaked syringe. These bone marrow stem cells were treated with 2-20% fetal bovine serum and 1-1,000 μM L-ascorbic acid-2-PO 4 , 5-15 ng / ml LIF (leukemia inhibitory factor) and 1-200 nM dexamethasone culture medium. All culture devices and slides were coated with 5 ng / ml collagen for 15 min at room temperature prior to receiving bone marrow stem cells. This in vitro condition allows bone marrow stem cells to maintain their self-renewal characteristics and expand through passage without loss of response to differentiation drugs such as growth factors. Moreover, this condition also allows bone marrow stem cells to maintain mesenchymal morphology and karyotype through subculture. figure 1 is a phase-contrast photomicrog...
Embodiment 2
[0074] Example 2: A method for inducing bone marrow stem cells to become cardiomyocytes with high cell incorporation rate and cell survival rate for a period of time
[0075] Bone marrow stem cells can be treated with biological growth factors in cardiac-specific medium for different periods of time, from 1 hour to 21 days, and during this period, about 40-90% of the cells express the nuclear protein MEF2. According to the different treatment time in heart-specific medium, the ratio of induced cardiomyocytes can be increased to 60-90%. The optimal treatment time can make 80-90% of the cells express MEF2 protein. Transplantation of 80-90% of these MEF2-expressing cells can optimally regenerate infarcted hearts.
[0076] Bone marrow stem cells isolated from adult dogs were cultured in cardiac-specific medium for 6 days, showing the optimal amount of MEF2 protein present in the nucleus under fluorescence microscopy. Transplantation of these cells into infarcted dogs showed inco...
Embodiment 3
[0079] Example 3: Transplantation of bone marrow stem cells and evaluation of cell incorporation rate and survival rate
[0080] Bone marrow stem cells differentiated in vitro into cardiomyogenic cell lines were transplanted into myocardial tissue of infarcted dogs. Myocardial infarction in dogs was produced by persistent occlusion of the left coronary artery. The infarction remained stable for at least 2 months prior to transplantation of bone marrow stem cells. To avoid immune rejection of the graft, bone marrow was collected from individual transplant recipient dogs and bone marrow stem cells were prepared as follows:
[0081] Approximately 4 weeks after ligation, after myocardial infarction was confirmed by echocardiography, bone marrow was aspirated after perforation on the ilium. Quickly mix the bone marrow and heparin, freeze and transfer to the tissue culture room with dry ice, where the bone marrow is thawed at 37°C, shaken, washed once with conventional DMEM, place...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com