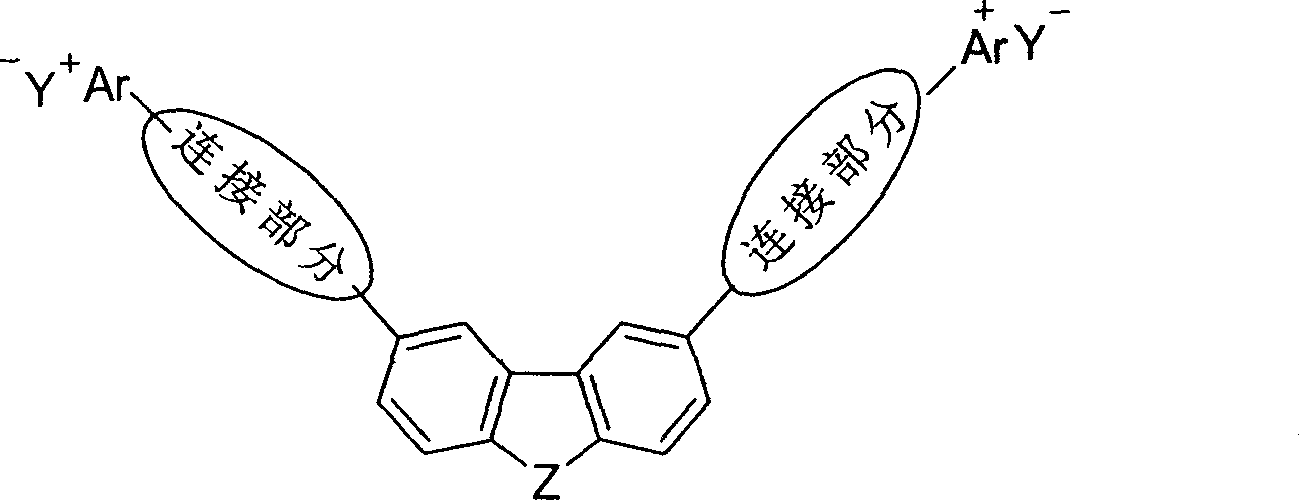
V-shaped conjugated light-absorbing organic salt compound and its use
A technology of organic salts and conjugated light, which is applied in the fields of organic polymer chemistry and material chemistry, can solve the problems that no V-type conjugated organic salt compounds have been reported, affect the macro-scale nonlinear optical response, and are difficult
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Synthesis of 3,6-bis[2-(N-methylpyridinium-4-ylvinyl)]-N-pentyl-9hydrocarbazole-di-p-toluenesulfonate:
[0085] The first step: the synthesis of 1,4-dimethylpyridinium p-toluenesulfonate:
[0086] Add 150mL of ethanol, 4.7g (50mmol) of 4-methylpyridine and 9.3g (50mmol) of methyl p-toluenesulfonate to the reaction vessel, heat and reflux for 6 hours, cool, evaporate most of the dissolved matter, and cool in an ice-water bath , filtered and dried to obtain 14.0 g of white crystals with a yield of 93%.
[0087] The second step: the synthesis of N-pentyl-9-hydrocarbazole-3,6-dialdehyde:
[0088] DMF 2.35mL (0.03mol) was placed in a three-necked flask, and cooled to below 10°C with a water bath. POCl 3 2.80 mL (0.03 mol) was slowly added dropwise into DMF using a constant pressure dropping funnel, and the temperature was controlled below 10°C. Dissolve 0.715 g (0.003 mol) of N-pentylcarbazole in dichloroethane, pour it into the reaction system, and raise the temperature...
Embodiment 2
[0092] Synthesis of 3,6-bis[2-(N-(thiazole-3-methyl)-pyridinium-4-ylvinyl)]-N-methyl-9-hydrocarbazole-dibromide:
[0093]The first step: the synthesis of 3,6-bis[2-(pyridin-4-yl)-vinyl]-N-methyl-9-hydrocarbazole
[0094] 1.625 g (5 mmol) of 3,6-dibromo-N-methyl-9-hydrocarbazole, 15.5 mg (0.06 mmol) of palladium acetate, and 150 mg (0.5 mmol) of tris(o-tolyl)phosphine were added to the reaction vessel. 2.10 g (20 mmol) of 4-vinylpyridine and 20 mL of triethylamine were refluxed for 24 hours under nitrogen protection, filtered after cooling, and the obtained solid was washed three times with water, then dissolved in THF, dried, filtered, and evaporated to remove the solvent. 1.10 g of orange solid was obtained with a yield of 59%.
[0095] The second step: 3,6-bis[2-(N-(thiazol-3-methyl)-pyridinium-4-ylvinyl)]-N-methyl-9-hydrocarbazole-dibromide Synthesis
[0096] Add ethanol 150mL, 3,6-bis[2-(pyridin-4-yl)-vinyl]-N-methyl-9-hydrocarbazole 3.87g (10mmol) and 3-bromomethylthia...
Embodiment 3
[0098] 3,6-bis[2-(N-(2,4-dinitrophenyl)-pyridinium-4-ylvinyl)]-N-methyl-9-hydrocarbazole-diiodide salt synthesis:
[0099] Add 50 mL of ethanol, 3,6-bis[2-(pyridin-4-yl)-vinyl]-N-methyl-9-hydrocarbazole 0.187 g (1 mmol) and 2,4-dinitrate into the reaction vessel 0.356 g (2 mmol) of iodobenzene was heated to reflux for 40 hours, cooled, and most of the solvent was evaporated, cooled in an ice-water bath, filtered, and dried to obtain 0.152 g of a dark red powder with a yield of 28%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com