Anion-binding polymers and uses thereof
A technology combining polymers and ions, applied in detergent compositions, surface active detergent compositions, chemical instruments and methods, etc., can solve problems such as side effects, discomfort, swelling, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0088] B. Polymer Preparation
[0089] The polymers of the present invention are prepared by methods well known to those skilled in the art. For example, ionically bound monomers or their precursors can be copolymerized in the presence of crosslinking agents; pre-synthesized ionically bound polymers are crosslinked by chemical reactions or radiation exposure; or polymer precursors are first Cross-linking, and further reacting on the polymer to generate ion-binding functional groups.
[0090] Polymers can be obtained by techniques such as direct or reverse suspension, emulsification, precipitation, polymerization in aerosol or by bulk polymerization / crosslinking methods, and size reduction processes such as extrusion and milling. Processes can be batch, semi-continuous and continuous.
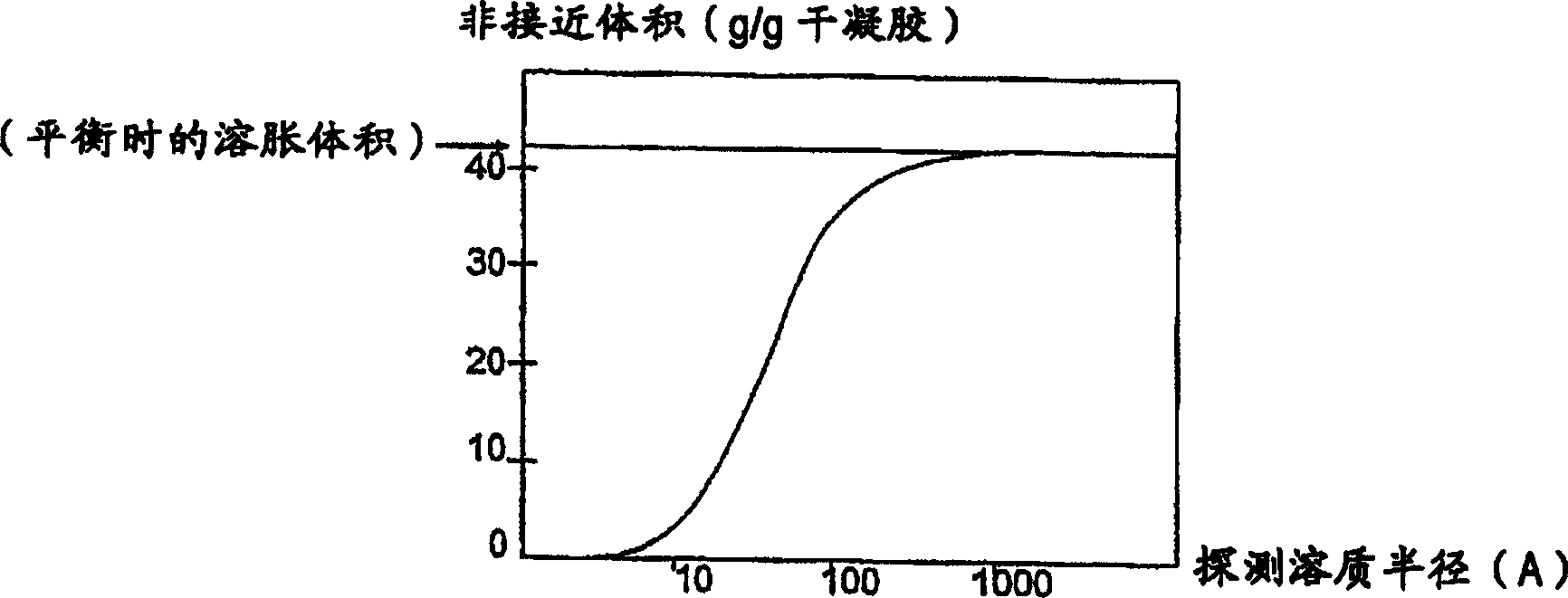
[0091] Swell rate, binding interference, binding capacity and molecular weight (MW) exclusion limit etc. will be affected by at least the following compositional and process variables:
[009...
Embodiment 1
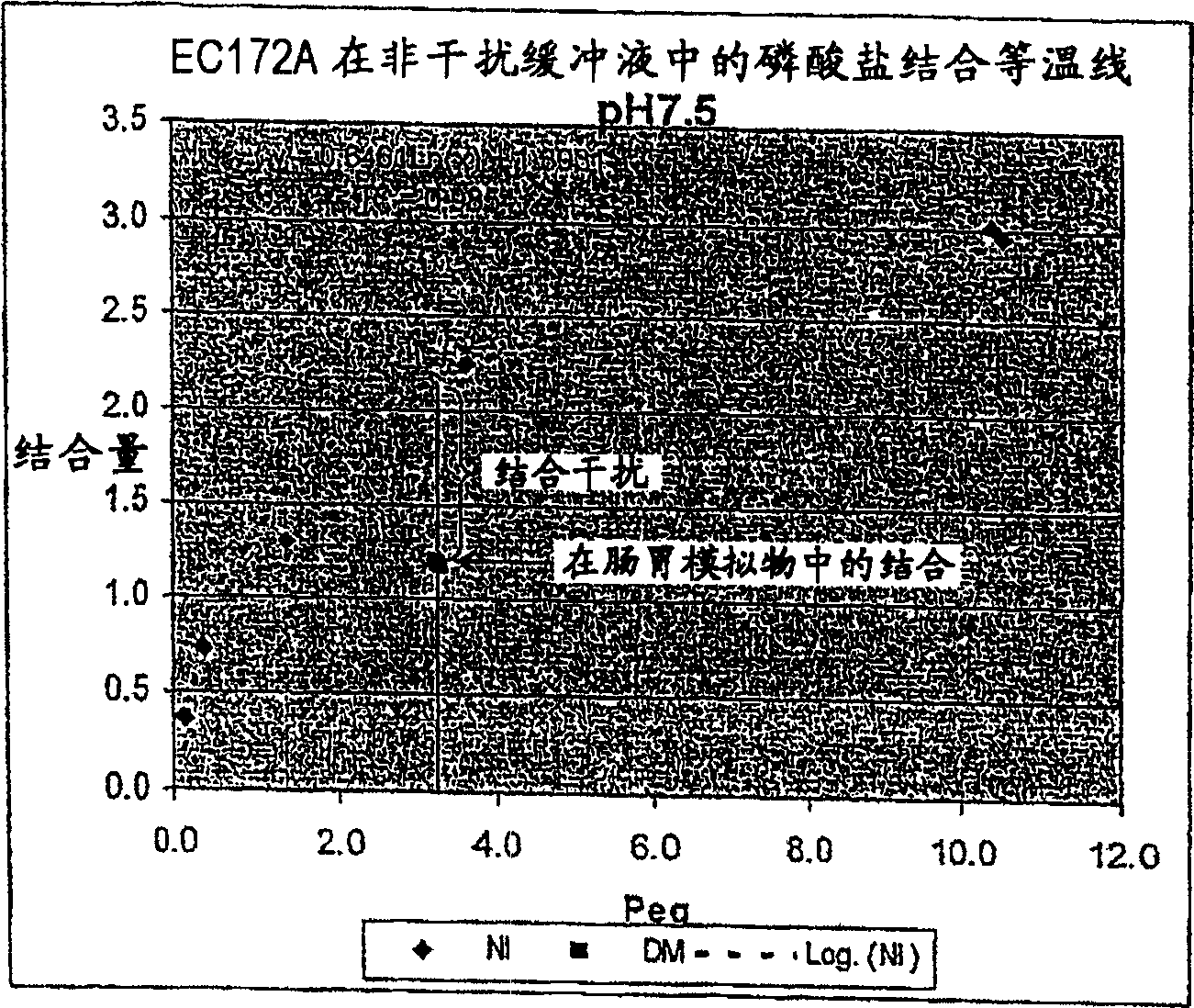
[0313] Embodiment 1: the assay method of phosphate binding capacity
[0314] This example describes various methods that can be used to determine the amount of anion incorporation in a polymer, where the anion is phosphate.
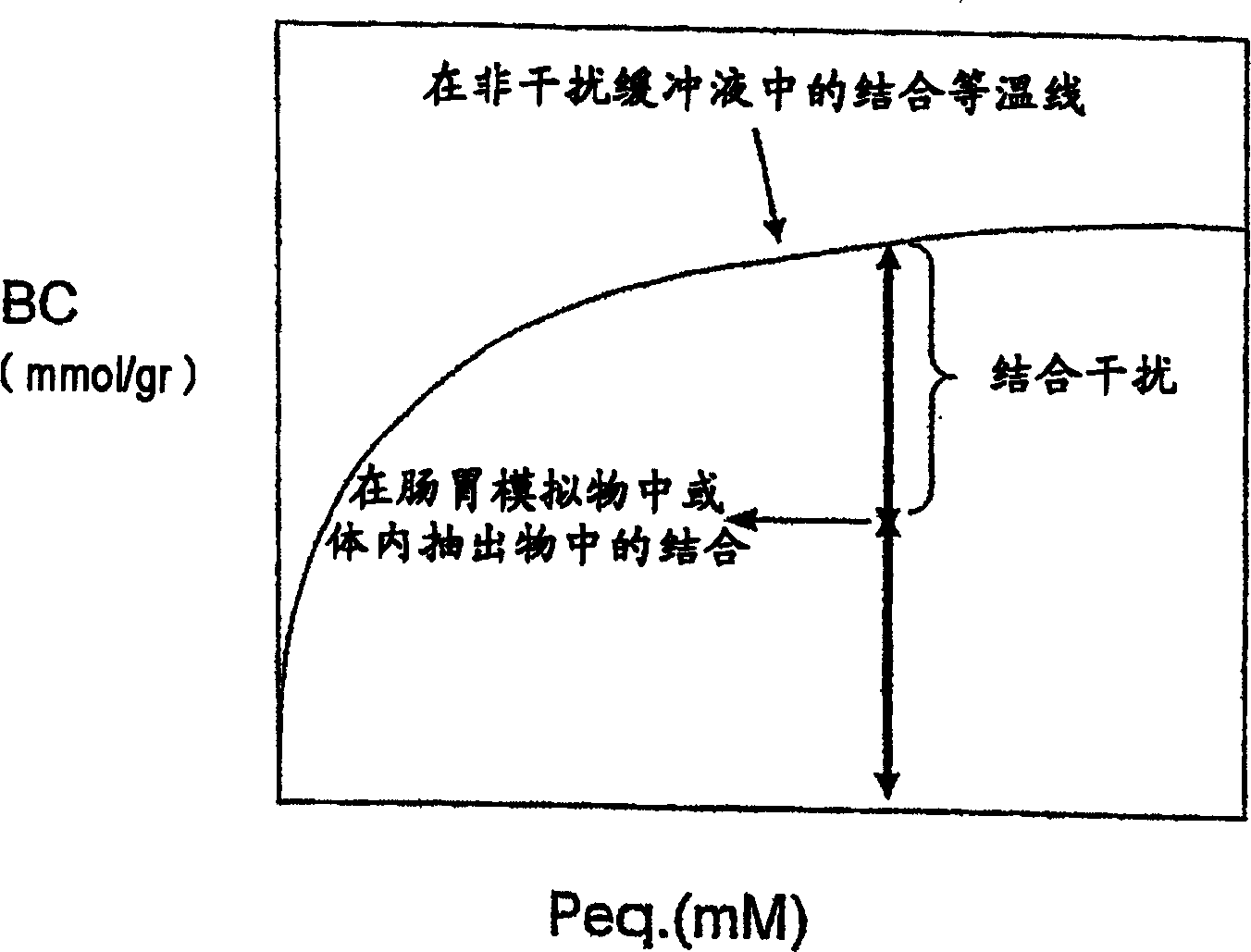
[0315] Determination of phosphate binding in non-interfering buffer
[0316] A sample amount of dry polymer P (gr) was mixed with a fixed volume V (L) of buffer containing 20 mM H 3 PO 4 , 80 mM NaCl, 100 mM MES sodium salt (morpholineethanesulfonic acid) and pH 6.5. This buffer can be used when performing a single binding assay. When performing multiple binding assays and drawing a binding isotherm, the concentration of buffer phosphate can be varied. The initial concentration of phosphate in the buffer is defined as P start (mM). This buffer is called a non-interfering buffer because it does not contain solutes that would compete with phosphate for binding to the polymer. After reaching equilibrium with the polymer, the solution was separated by ...
Embodiment 2
[0327] Example 2: Generation of cross-linked polymer libraries using bulk solution method and determination of phosphate incorporation to generate polymer libraries
[0328] The following five examples each include one polymer library, and each polymer library contains up to 24 cross-linked polymers. These polymers were synthesized using batch reactors arranged in a 4 x 6 array, each reactor was magnetically stirred with temperature control, and had a volume of 350 microliters or 3 milliliters. In a typical procedure, first, the amine, the crosslinker and the solvent and possibly the base are added to the reactor by a robot with optional agitation. The reactor was then sealed and heated to the specified temperature for 15 hours. The reactor array was then removed and the crosslinked polymer mass transferred to a glass vial. After crushing, it was repeatedly washed with deionized water, and then freeze-dried. Table 3 shows the five polymer libraries and their corresponding s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com