Process for synthesizing antithrombin inhibitor of non-asymmetric non-peptide kind
An antithrombin, non-peptide technology, applied in the field of synthesis of new antithrombin inhibitors, can solve the problems of high price, low total yield, low yield, etc. high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
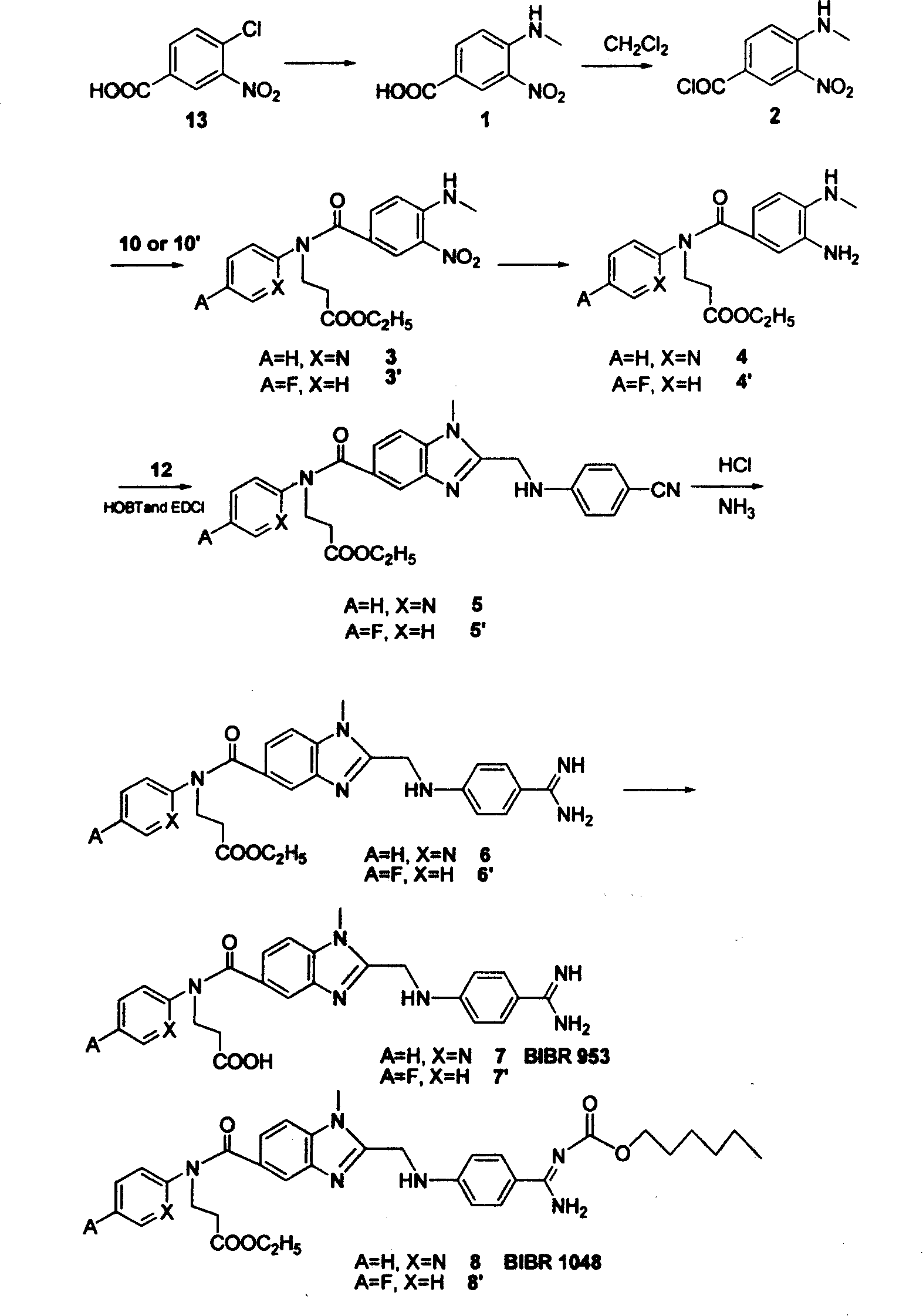
Embodiment 1
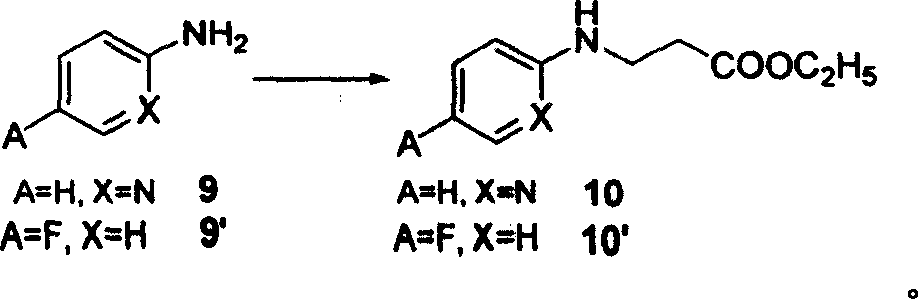
[0039] Synthesis of ethyl 3-(pyridine-2-imine)-propionate (10)
[0040]Under nitrogen protection, add ethyl acrylate (27.5 g, 0.275 mol) to compound 9 (A=H, X=N)-2-aminopyridine (22.5 g, 0.25 mol), and stir and reflux at a temperature higher than 100 ° C for 24 h , the precipitate was filtered off, and the residue was concentrated and purified by a silica gel column to obtain a white solid 10 (38 g, 72%).
[0041] Synthesis of 1-(4-cyano-phenylimine)-acetic acid (12)
[0042] Add 150ml of water to compound 11 (6.0g, 0.05mol) and 1-chloroacetic acid (10g, 0.11mol) and heat to reflux until a large amount of yellow solid precipitates, filter at room temperature, and rinse with water, absolute ethanol, and anhydrous ether, respectively. After washing, yellow solid 12 (6.4 g, 73%) was obtained.
[0043] Synthesis of 4-aminomethyl-3-nitro-benzoic acid (1)
[0044] 150ml of 25%-30% methylamine aqueous solution was added to compound 13 (25g, 0.124mol), and the system was reacted at...
Embodiment 2
[0060] Synthesis of ethyl 3-(4-fluorophenyl-1-imine)-propionate (10')
[0061] Under nitrogen protection, ethyl acrylate (27.5 g, 0.275 mol) was added to compound 9'(A=F, X=H)-4-fluoroaniline (23.5 g, 0.25 mol), 10 ml of absolute ethanol and 10 ml of Tris Ethylamine was stirred and refluxed at higher than 100°C for 24h, the precipitate was filtered off, and the residue was concentrated and purified by a silica gel column to obtain a light red solid 10' (43g, 77%).
[0062] Synthesis of 3-[(4-aminomethyl-3-nitro-benzoyl)-(4-fluorobenzene)-2-imine]-propionic acid ethyl ester (3')
[0063] Compound 10' (10.5g, 0.05mol) was dissolved in 30ml CH 2 Cl 2 and 30ml triethylamine, slowly add the CH of compound 2 at room temperature 2 Cl 2 solution. The mixed system was reacted at room temperature for 12 h, the precipitate was filtered off, and the residue was concentrated and purified by a silica gel column to obtain a yellow oily liquid 3' (19.0 g, 98%).
[0064] Synthesis of 3-[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com