PcrDNA vaccine, and its preparing method and use
A vaccine and universal primer technology, applied in the biological field, can solve the problems of reducing the immune effect, harming the body, reducing the immune efficiency of DNA vaccines, etc., and achieve the effects of good biological safety, safe use and high biological safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
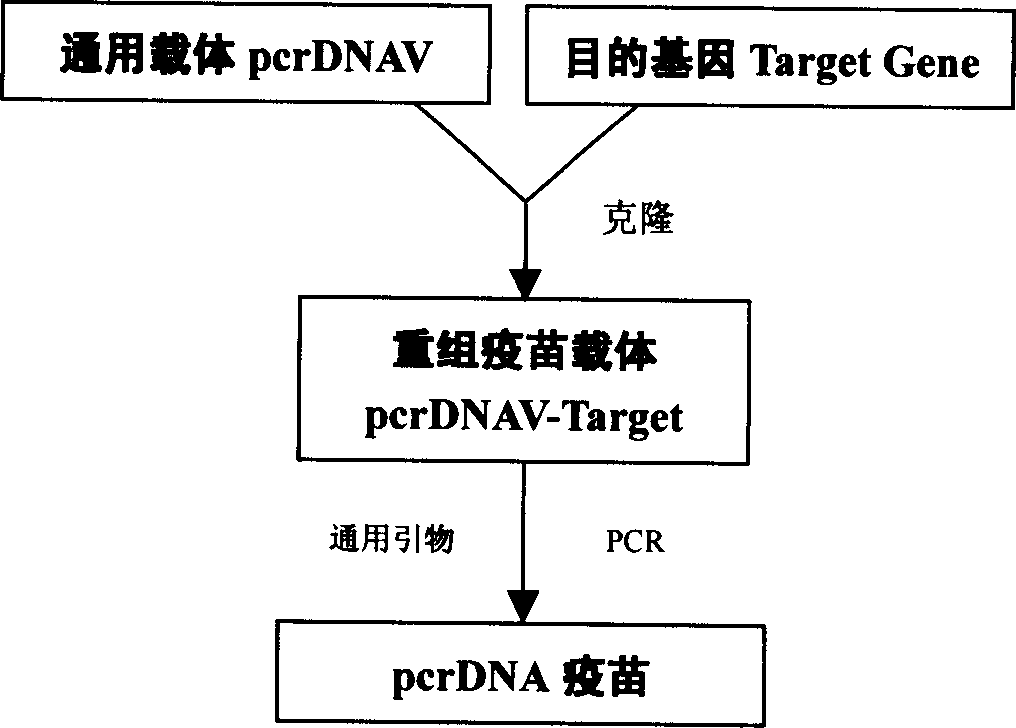
[0036] Example 1 Construction of pcrDNA vaccine universal vector pcrDNAV (such as figure 1 Shown)
[0037] Based on the pMD 18-T vector, the human cytomegalovirus promoter (CMV) and the PolyA tail of simian virus 40 (SV40) were added at the multiple cloning site to construct a universal vector pcrDNAV ( Such as image 3 Shown).
[0038] 1. The CMV promoter and SV40 PolyA were cloned into the pMD 18-T vector to construct the PCRDNA vaccine universal vector pcrDNAV.
[0039] (1) Design of primers for amplification of CMV and SV40 PolyA fragments
[0040] According to the pAdTrack-CMV gene sequence in GenBank, a pair of primers were designed for each of the CMV promoter and SV40 PolyA gene. The primers were synthesized by Shanghai Boya Biotechnology Co., Ltd.
[0041] CMV upstream primer P1: 5’-CGGAATTCATTCTTTCCCACCCTTA-3’ (contains EcoR I restriction site)
[0042] CMV downstream primer P2: 5’-TTGGTACCAGATCTCTAGCGGATC-3’ (contains KpnI, BgI II restriction sites)
[0043] SV40 PolyA...
Embodiment 2
[0050] Example 2 Design of universal primers for amplifying PCRDNA vaccine from PCRDNAV vector
[0051] Design the universal primers for the amplified vaccine at the upstream of the CMV promoter and downstream of the SV40 PolyA of the constructed pcrDNAV vaccine universal vector:
[0052] Upstream primer: 5’-ACAGCTATGACCATGATTACG-3’
[0053] Downstream primer: 5’-AACGACGGCCAGTGCCAAG-3’
Embodiment 3
[0054] Example 3 General process for the construction of pcrDNA vaccine
[0055] Design primers to amplify the desired target gene by PCR or use enzyme digestion method, according to the conventional gene cloning method (enzyme digestion method or PCR amplification method) cloned into the pcrDNA vector (high-efficiency PCR where the PCR product is directly connected to the pcrDNA vector The product ligation site or ligation of the digested product into the multiple cloning site of the PCRDNA vector), the recombinant plasmid pcrDNA-Target is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com