High secretion expression of recombination thymosin-alpha 1 in Escherichia coli and separation and purification therefor
A technique of secreting and expressing Escherichia coli, which is applied in the field of bioengineering and can solve problems such as cumbersome and complex production processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Design and synthesis of recombinant thymosin α1 gene:
[0044] We designed the following two fragments (SEQ ID NO.3 and SEQ ID NO.4 in the sequence listing), the underlined part is the complementary region of the two fragments. When designing the synthetic fragments, the following principles were fully followed: according to the amino acid sequence of natural thymosin α1 (SEQ ID NO.10 in the sequence listing), the preferred codons of Escherichia coli were selected; the AT and GC contents were close and evenly distributed; the fragments To avoid the generation of secondary structure within; to avoid repeated sequences between fragments; to add two stop codons at the 3' end of the gene.
[0045] Fragment 1: 5′-TCTGACGCTGCTGTTGACACTTCTTCCGAAATCACTAC
[0046] CAAA GACCTGAAAGAAAAG -3′
[0047] Fragment 2: 5′-CCCAAGCTTATTAGTTTTCAGCCTCTTCTACAACTTCTTT
[0048] CTTTTCTTTCAGGTC -3′
[0049] Each of the two chemically synthesized gene fragments was 10D, ...
Embodiment 2
[0050] Example 2 Chemical synthesis of alkaline phosphatase phoA promoter and signal peptide gene:
[0051] We synthesized the gene completely according to the natural gene sequence (SEQ ID NO.5 in the sequence listing) of Escherichia coli alkaline phosphatase phoA promoter (phoA-P) and signal peptide (sig). The following four fragments (fragments 3-6) were first synthesized (SEQ ID NO.6, SEQ ID NO.7, SEQ ID NO.8 and SEQ ID NO.9 in the sequence listing), and the underlined part is the complementary region between the fragments. Add PstI, BglII, and XbaI restriction sites at the 5' end of phoA-P to facilitate construction.
[0052] Fragment 3: 5′-AACTGCAGATCTAGAGCTCGTCAGTAAAAAGTTAATCTTT
[0053] TC AACAGCTGTCATAAAGTT -3'
[0054] Fragment 4: 5'- TTAAAAAATAAAAACAAA GCGACTATAAGTCTCGGCCGTG
[0055] AC AACTTTATGACAGCTGTT -3'
[0056] Fragment 5: 5'- TTTGTTTTTTATTTTTAA TGTATTTGTACATGGAGAAAAT
[0057] AAA AGTGAAAACAAAGCACTAT -3'
[005...
Embodiment 3
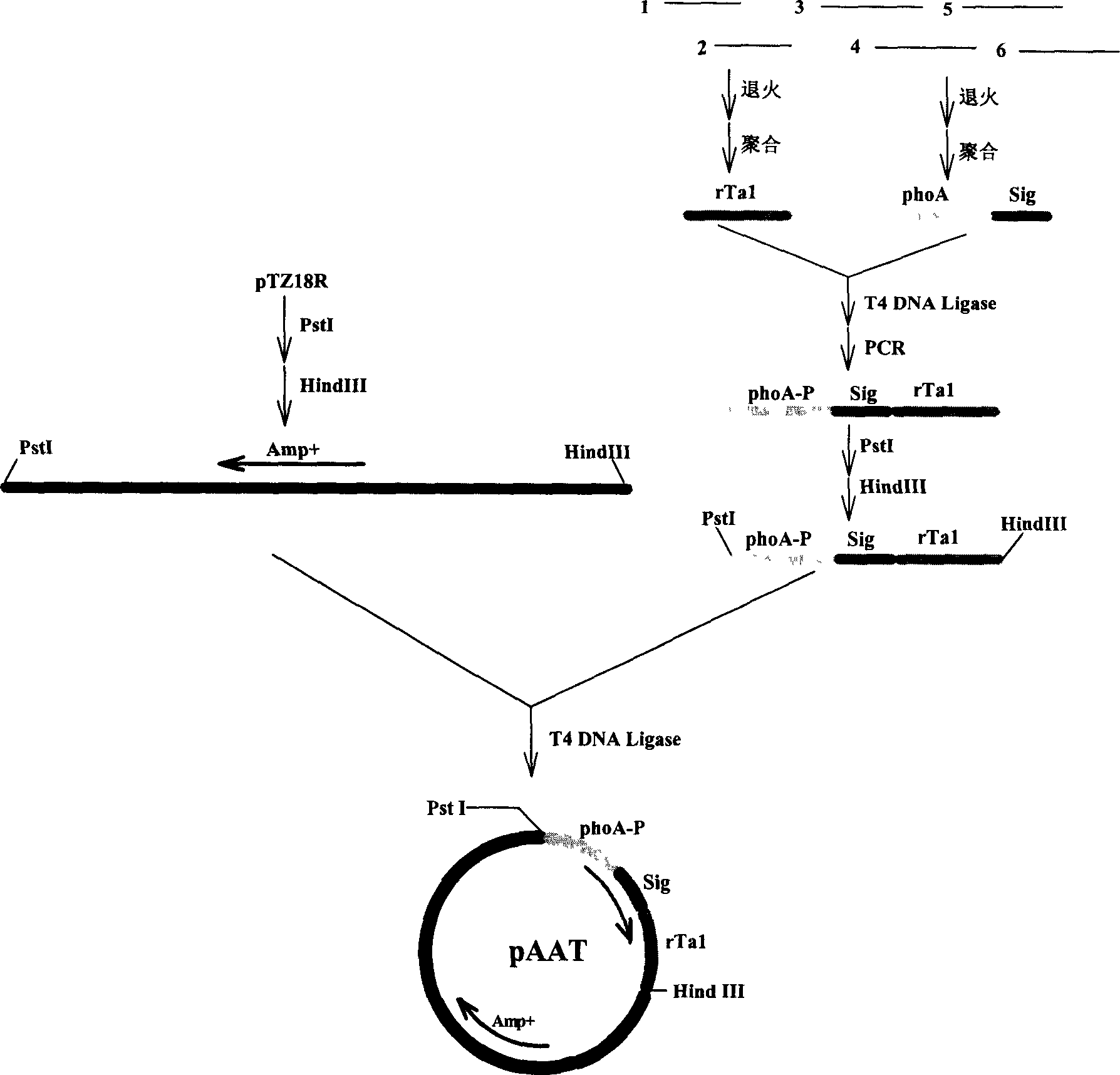
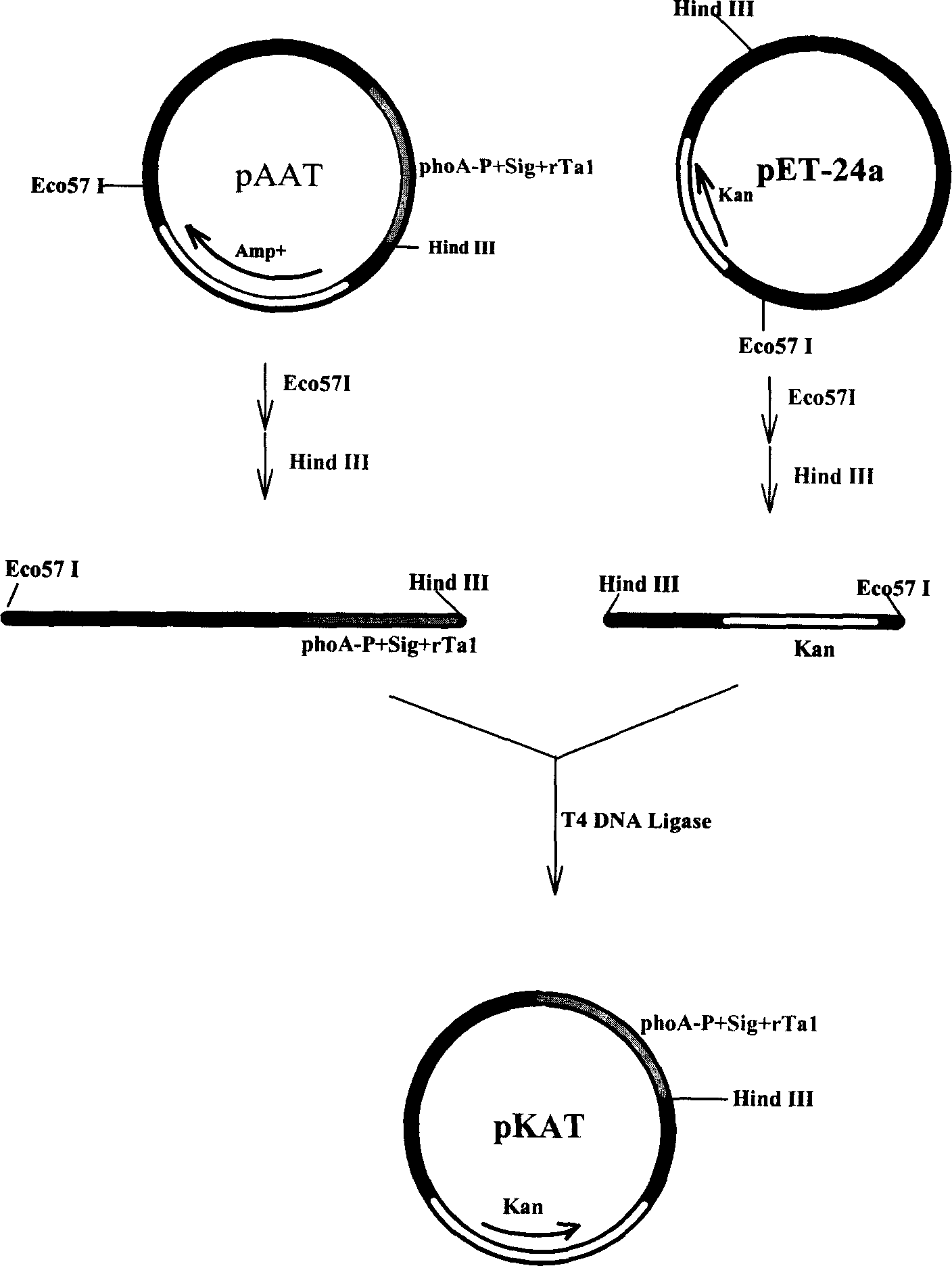
[0060] Example 3 Construction of expression plasmids ( figure 1 ):
[0061] 3.1 Enzyme digestion treatment of vector:
[0062] Plasmid pTZ18R (Pharmacia Company) was used as a starting plasmid. Take 5 μg of pTZ18R, add 20 UPstI enzyme (TaKaRa company) and 10 μl 10×PstI enzyme buffer (TaKaRa company), add distilled water to 100 μl, digest at 37°C for 4 hours, then add 10 μl 3M sodium acetate, 200 μl absolute ethanol, fully Mix well, centrifuge at 12000rpm for 10 minutes, discard the supernatant, add 200 μl of 70% ethanol, mix well, centrifuge at 12000rpm for 2 minutes, discard the supernatant. After the precipitate was dried, add 20U HindIII enzyme (TaKaRa Company) and 5 μl 10×HindIII enzyme buffer (TaKaRa Company), supplement distilled water to 50 μl, digest overnight at 37°C, and electrophoresis the digestion mixture in 1% agarose gel. Cut out a band of about 2.9 kb, use the DNA gel recovery kit (Shanghai Sangon Bioengineering Technology Service Co., Ltd.), recover the DNA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com