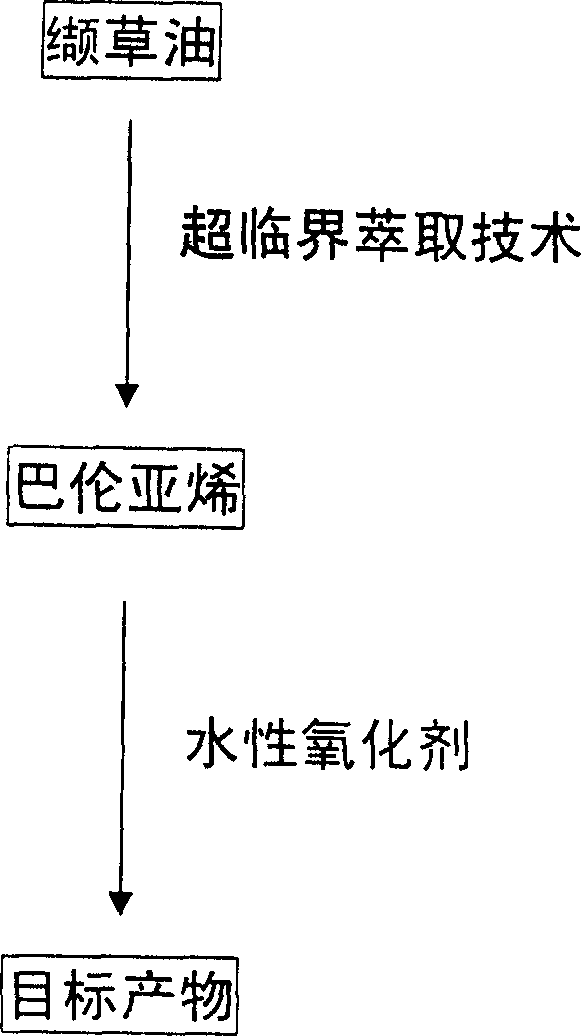
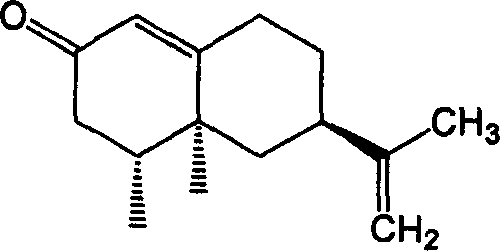
Method for synthesizing nootkatone, and its application
A synthesis method and technology of nokatone, which is applied in the field of synthesis of unsaturated ketene compounds, can solve the problems of low content, limited dosage, difficulty in isolation, etc., and achieve the effects of good selectivity, rich cigarette flavor, and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] (2) Mix 15g of balentene, 300ml of ethyl acetate, 4g of sodium bicarbonate and 60g of tert-butyl hydroperoxide (TBHP), and add the balun 10% aqueous solution of sodium hypochlorite in an equimolar amount of alkene, after the mixture was stirred for 13 hours, 50 g of sodium bisulfite was added, and after standing for 6 hours, the water layer was separated into layers. %NaHCO 3 Wash, wash with water three times, add 10g of anhydrous sodium sulfate to dry for 20h, distill off ethyl acetate to obtain 16.2g of nokadone primary product, carry out vacuum distillation on the primary product, and collect fractions at 185~195°C / 1300Pa. The obtained product was separated by a silica gel column (V (n-hexane) / V (ether) = 3 / 1), and the component with Rf = 0.51 was collected, and 13.1 g of colorless needle crystals were obtained after low-temperature recrystallization. Yield 82%, melting point 34.5~35.5℃, optical rotation [α] D 25 =+189.5°(c 0.50, CH 3 OH), refractive index...
Embodiment 2
[0020] (2) Mix 10g of balentene, 200ml of ethyl acetate, 4g of sodium bicarbonate and 60g of tert-butyl hydroperoxide (TBHP), and add the balun 10% aqueous solution of sodium hypochlorite in an equimolar amount of alkene, after the mixture was stirred for 4 hours, 45 g of sodium bisulfite was added, and after standing for 3 hours, the water layer was separated into layers, and the water layer was extracted three times with ethyl acetate, and the organic layers were combined, and the organic layer was washed with 10 %NaHCO 3 Washing, washing with water three times, adding 10g of anhydrous sodium sulfate to dry for 20h, distilling off ethyl acetate to obtain 12.3g of nokadone primary product, the primary product was subjected to vacuum distillation, and the fraction at 185~195°C / 1300Pa was collected. The obtained product was separated by a silica gel column (V (n-hexane) / V (ether)=3 / 1), and the component with Rf=0.51 was collected, and 8.8 g of colorless needle crystals w...
Embodiment 3
[0025] (2) Mix 20g of balentene, 400ml of ethyl acetate, 8g of sodium bicarbonate and 80g of tert-butyl hydroperoxide (TBHP), and add the balun 10% aqueous solution of sodium hypochlorite in an equimolar amount of alkene. After the mixture was stirred for 15 hours, 55 g of sodium bisulfite was added. After standing for 12 hours, the water layer was separated into layers. The water layer was extracted three times with ethyl acetate, and the organic layers were combined. %NaHCO3 Washing, washing with water three times, adding 15g of anhydrous sodium sulfate to dry for 24h, distilling off ethyl acetate to obtain 24.5g of nokadone primary product, the primary product was subjected to vacuum distillation, and the fraction at 185~195°C / 1300Pa was collected. The obtained product was separated by a silica gel column (V (n-hexane) / V (ether) = 3 / 1), and the component with Rf = 0.51 was collected, and 16.9 g of colorless needle crystals were obtained after low-temperature recrystal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com