Methods and compositions for detecting SARS virus and other infectious agents
A technology of viruses and coronaviruses, applied in biochemical equipment and methods, measurement/testing of microorganisms, resistance to vector-borne diseases, etc., can solve the problem that RT-PCR cannot meet the needs of early clinical screening and diagnosis, RT-PCR detection Low rate, expensive and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0227] Example 1. Probe design
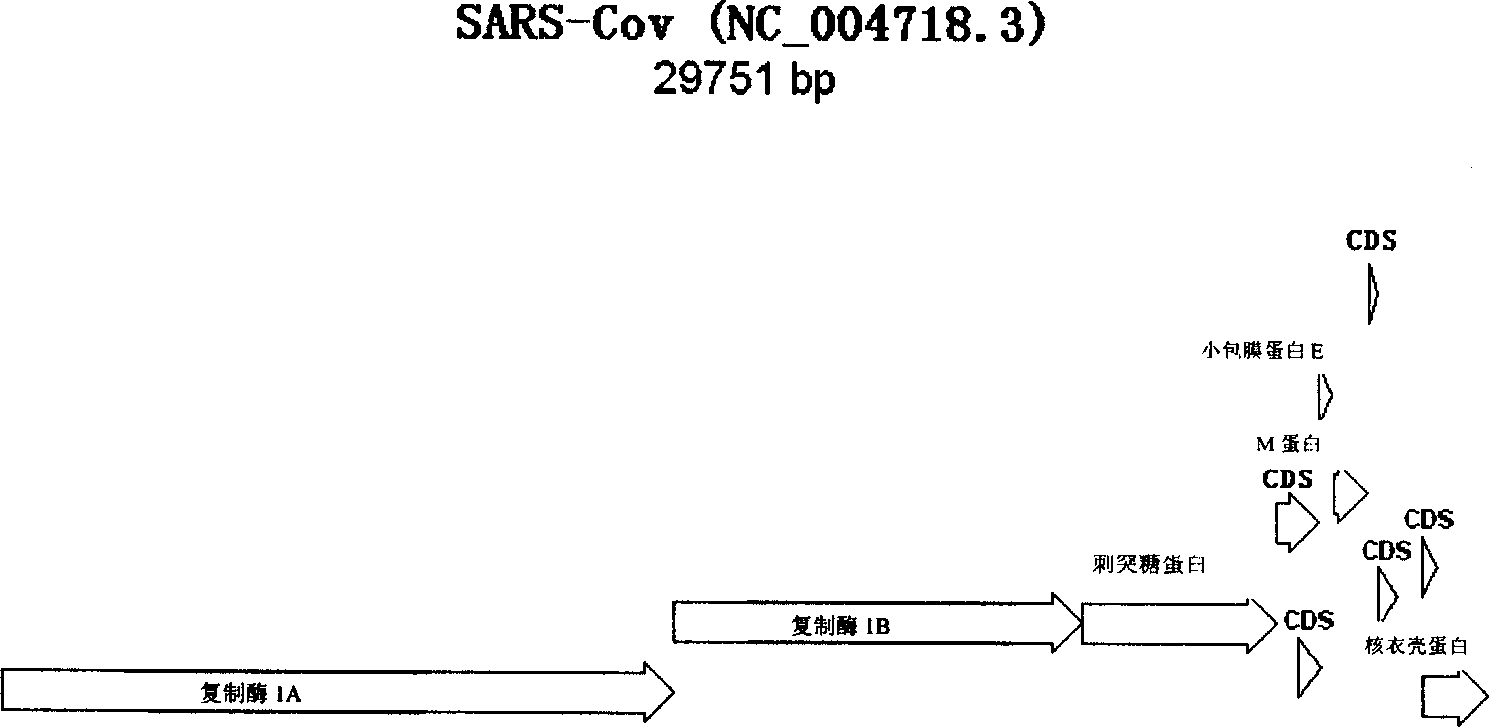
[0228] Various genomic sequences of SARS-CoV are available (see Table 22 for example).
[0229] serial number
SARS crown
Source
Country of submission
Home (region)
Acc
N in the sequence
Number of
Genome leader
Degree
Ratio of N
example
SARS_BJ01
Beijing China
China
AY278488
900
28920
3.11%
SARS_BJ02
Beijing China
China
AY278487
300
29430
1.02%
SARS_BJ03
Beijing China
China
AY278490
607
29291
2.07%
SARS_GZ01
Beijing China
China
AY278489
1007
29429
3.42%
SARS_BJ04
Beijing China
China
AY279354
2502
24774
10.10%
SARS_
CUHK-W1
China Hong Kong
China Hong Kong
AY278554
0
29736
0.00%
SARS_HKU-398
49
China Hong Kong
...
Embodiment 2
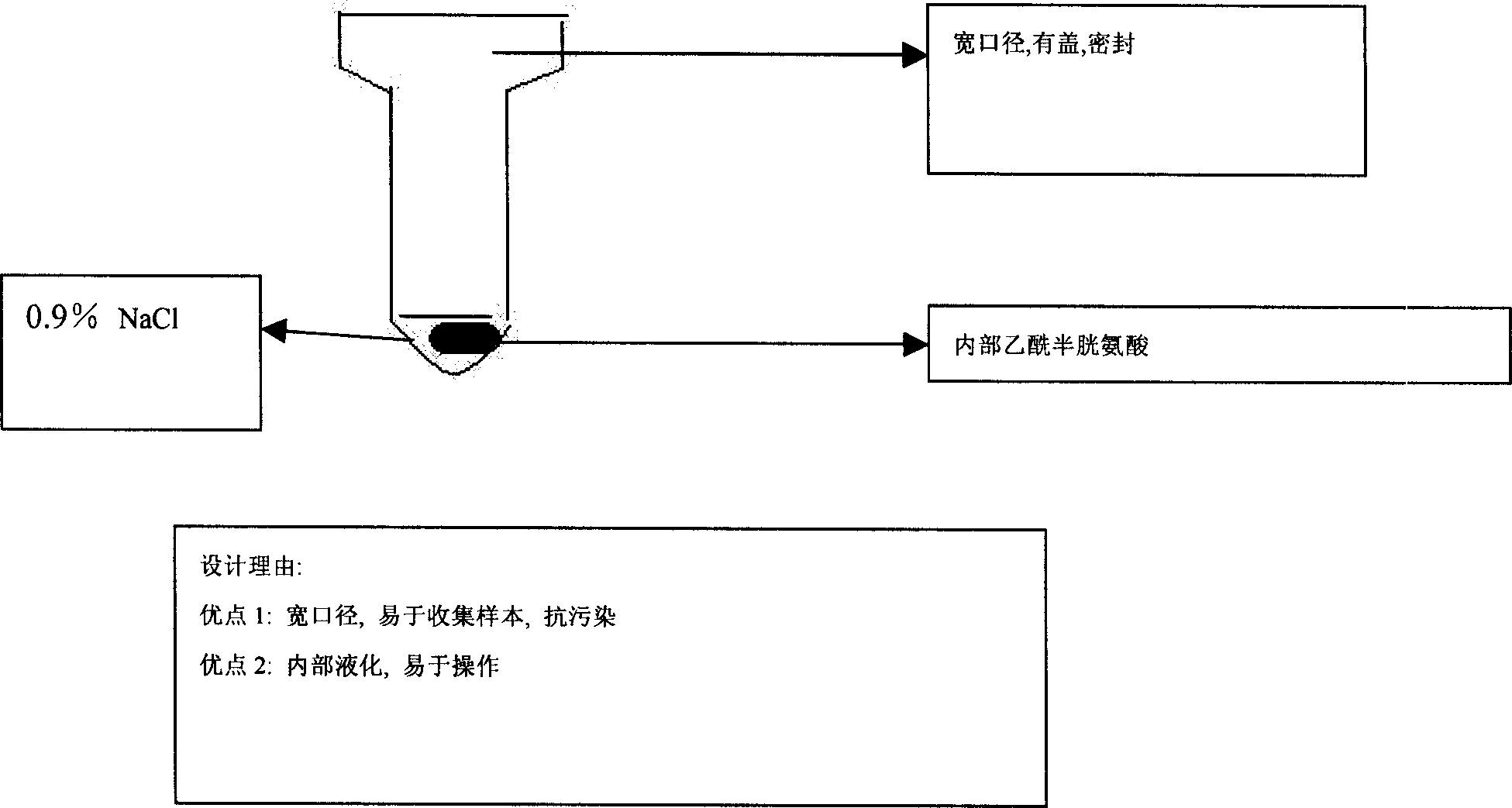
[0243] Example 2: Pre-processing of blood samples
[0244]The pre-processing of blood samples involves a relatively complicated process. However, considering the relatively low SARS virus concentration reported in serum, the pretreatment described here can effectively enrich lymphocytes from approximately 2 ml of whole blood in order to increase the chance of detection.
[0245] 1. Sample collection and transmission
[0246] 1) Collect samples from patients in the hospital and put them into the first transmission window. Then close and lock the door of the transmission window.
[0247] 2) Then transfer the sample to the second transmission window. Record the sample in the notebook and print 3 barcode labels. Then the sample is routinely tested and transferred to the pre-processing transmission window.
[0248] 2. Use a biological safety cabinet
[0249] 1) The hospital staff performing the pre-processing process enter the pre-processing studio and close the door. Open the bi...
Embodiment 3
[0277] Example 3. The process of using QIAamp Viral RNA kit to extract RNA
[0278] The following steps are used in the RNA preparation process:
[0279] 1. Use a pipette to add 560μl of the prepared buffer AVL containing Carrier RNA to a 1.5ml microcentrifuge tube. If the sample volume is greater than 140 μl, increase the amount of buffer AVL / Carrier RNA in the same proportion (for example, a 280 μl sample will require 1120 μl of buffer AVL / Carrier RNA).
[0280] 2. Add 140μl of plasma, serum, urine, cell culture supernatant or cell-free body fluid to the buffer AVL / Carrier RNA in the microcentrifuge tube. Mix for 15 seconds with a pulse vortex shaker. To ensure effective lysis, it is important to thoroughly mix the sample with buffer AVL to obtain a uniform solution. Frozen samples that have only been thawed once can also be used.
[0281] 3. Incubate for 10 minutes at room temperature (15-25°C). The virus particles can be completely lysed after 10 minutes at room temperature....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com