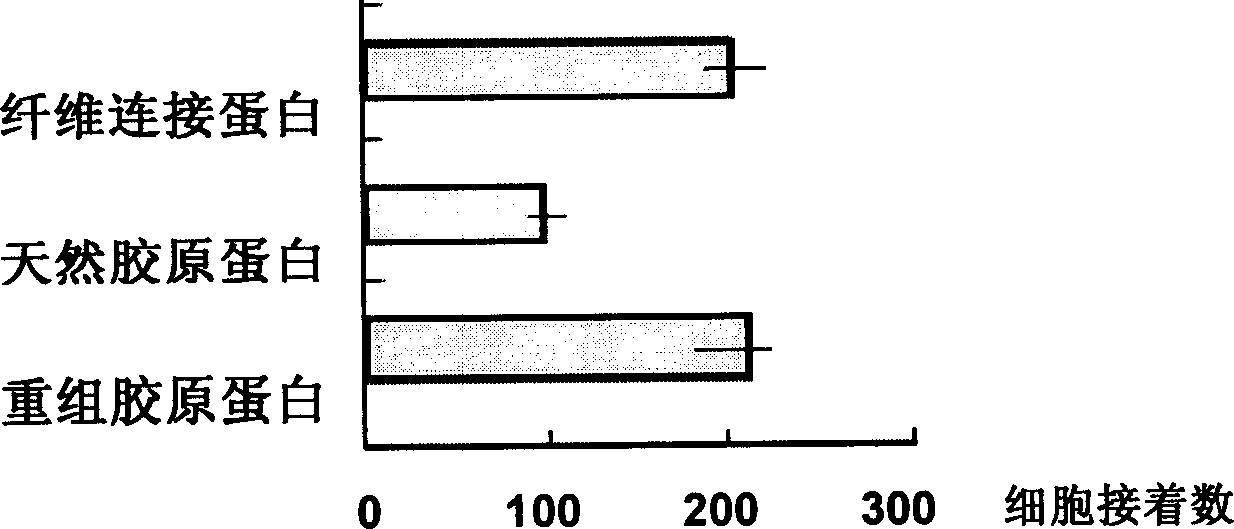
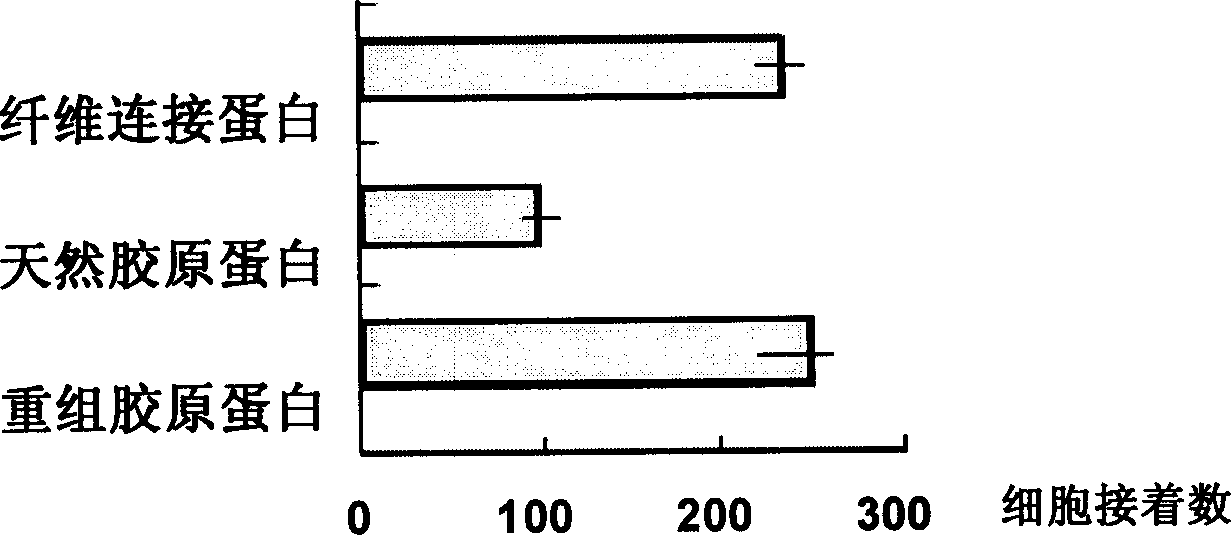
Recombined collagen and synthesizing and expressing purifying process thereof
A technology of recombinant collagen and synthetic method, applied in the direction of recombinant DNA technology, animal/human protein, connective tissue peptide, etc., can solve the problem of inability to form hydroxyproline residues and inability to synthesize proline-4-hydroxylase and other issues to achieve the effect of low immune rejection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1, the construction of recombinant collagen:
[0051] The amino acid sequence of a designed recombinant collagen is as follows:
[0052] [Gly-Glu-Arg-Gly-Asp-Leu-Gly-Pro-Gin-Gly-Ile-Ala-Gly-Gln-Arg-Gly-Val-Val-Gly-Glu-Arg-Gly-Glu-Arg-Gly -Glu-Arg-Gly-Ala-Ser]8-Gly-Pro-Pro-Gly-Pro-Cys-Cys-Gly-Gly-Gly
[0053] (1) Designed and synthesized four oligonucleotides: Coll-1, Coll-2, Fusion-1 and Fusion-2, and then used the annealing method to obtain two double-helical DNAs respectively, and their nucleotide sequences were respectively for:
[0054] Coll-1:
[0055] 5' CTAGTGGCGAACGTGGTGATCTGGGCCCGCAGGGTATCGCGGGCCAGCGTGGTGTGGTTGGCGAGCGTGGTGAACGCGGCGAGCGTGGTG 3'
[0056] Coll-2:
[0057] 5' CTAGCACCACGCTCGCCGCGTTCACCACGCTCCCGAACCACACCACGCTGGCCCGCGATACCCTGCGGGCCCAGATCACCACGTTCGCCA 3'
[0058] Fusion-1:
[0059] 5'CTAGTGGCCCGCCAGGTCCGTGCTGTGGCGGTGGCG 3'
[0060] Fusion-2:
[0061] 5'CTAGCGCCACCGCCACAGCACGGACCTGGCGGGCCA 3'
[0062] Coll-1 and Coll-2 obtained by ...
Embodiment 2
[0078] Embodiment 2, the expression of recombinant collagen protein:
[0079] (1) Transform the competent cell BL21(DE3)pLysS (purchased from Novagen) bacteria with the pET30a(+)-Coll(8)-Fusion plasmid verified by sequencing, pick a single colony and inoculate it in LB liquid medium, and culture it with shaking at 37°C overnight, and then inoculated in TB liquid medium containing 10-50 μg / ml kanamycin and 25-170 μg / ml chloramphenicol at a ratio of 1:100, when OD 600 When reaching about 0.5-1.0, add inducer IPTG to induce protein expression, and the concentration of IPTG is 0.1-2mM. After continuing to cultivate for 2-5 hours, the bacterial cells were collected by centrifugation (5000×g, 4° C. for 10 minutes).
[0080] The special feature of the present invention is that the time and temperature for inducing expression can be adjusted according to the actual situation, such as the solubility, stability and expression level of the target protein, which can be adjusted to 30°C f...
Embodiment 3
[0081] Embodiment 3, the purification of recombinant collagen protein:
[0082] The bacteria collected above were resuspended with ice-cold cell lysis buffer (Cell Lysis Buffer) 3-7 times the wet weight of the cells, placed in an ice bath, and the cells were ultrasonically disrupted to release the protein Coll(8)-Fusion. After the cells are broken, the viscosity of the liquid increases. If the viscosity of the mixed solution is too high, the cell lysate can be diluted appropriately, or MgCl with a final concentration of 5mM can be added. 2 10 μg / ml of protease-free DNase to reduce viscosity. Centrifuge at 20,000×g at 4°C for 30 minutes, and the supernatant is the clarified crude cell extract. The crude extract was subjected to metal chelation chromatography, dialyzed in distilled water, and then freeze-dried to collect the protein. Protein expression and purification results were confirmed by SDS-PAGE gel electrophoresis and Western blotting.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com