Therapy for primary and metastatic cancers
A functional, nasopharyngeal carcinoma technology, applied in the direction of fermentation, unknown raw materials, botanical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
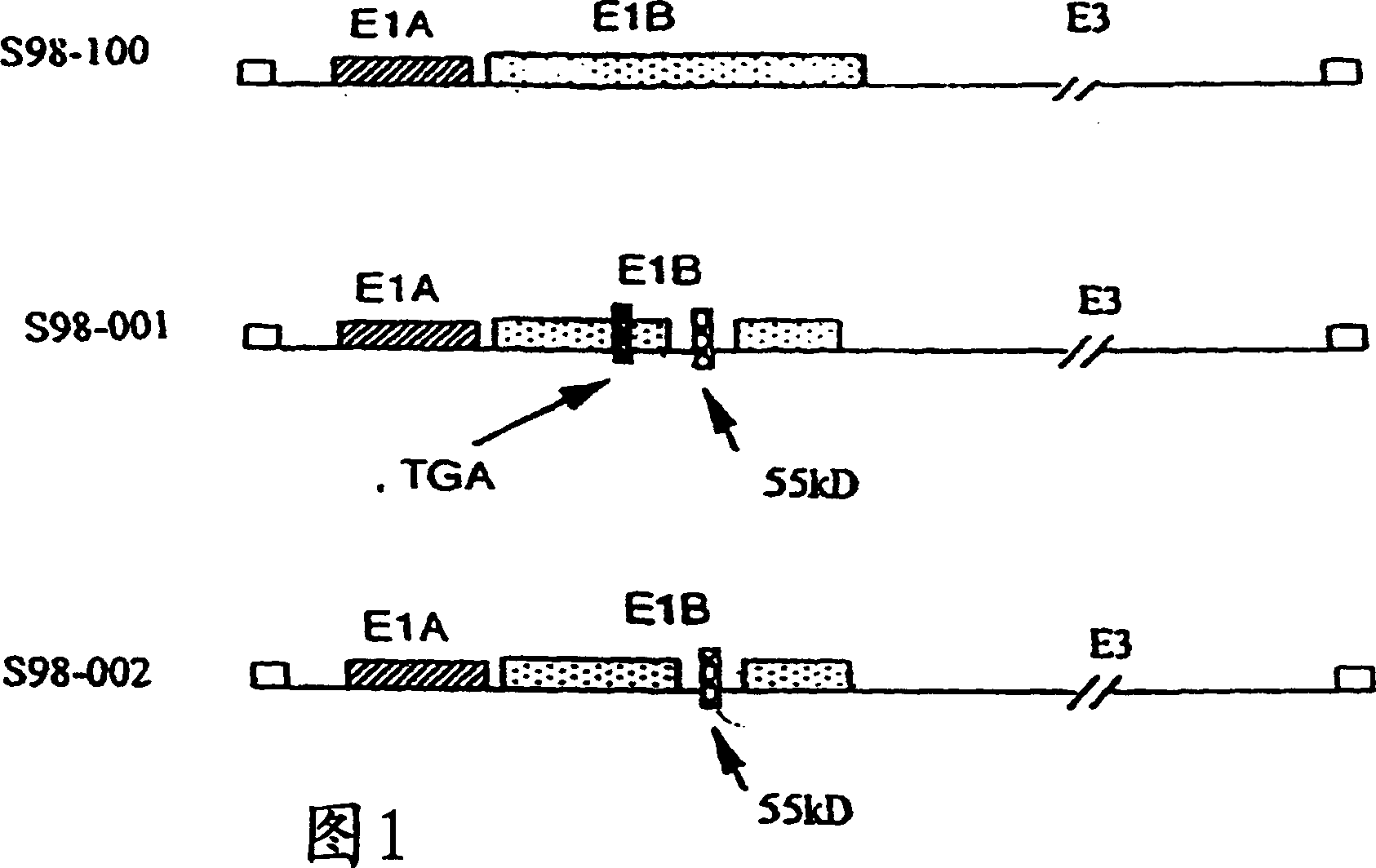
[0076] Adenovirus is usually a double-stranded DNA genome virus that causes respiratory, intestinal, and eye infections in humans or animals. The virus that causes the common cold is an adenovirus. The oncolytic virus of the present invention includes genetically engineered adenovirus Ad5 variants. The present invention uses specific genetic engineering variants of the Ad5 virus, including S98-001 (SeqID#1) or S98-002 (SeqID#2). Although not wanting to be bound by theory, it is well known that human infection with wild-type Ad5 can heal itself. In addition, Ad5 adenovirus has been routinely used as a gene therapy vector because there is no report that DNA fragments of the Ad5 genome can be integrated into the genome of human cells. Therefore, the present invention also adopts the synchronization of injection of specific oncolytic virus and high temperature to inhibit cancer at the injection site and far away from the virus injection site. Although oncolytic Ad5 variants are used a...
Embodiment 2
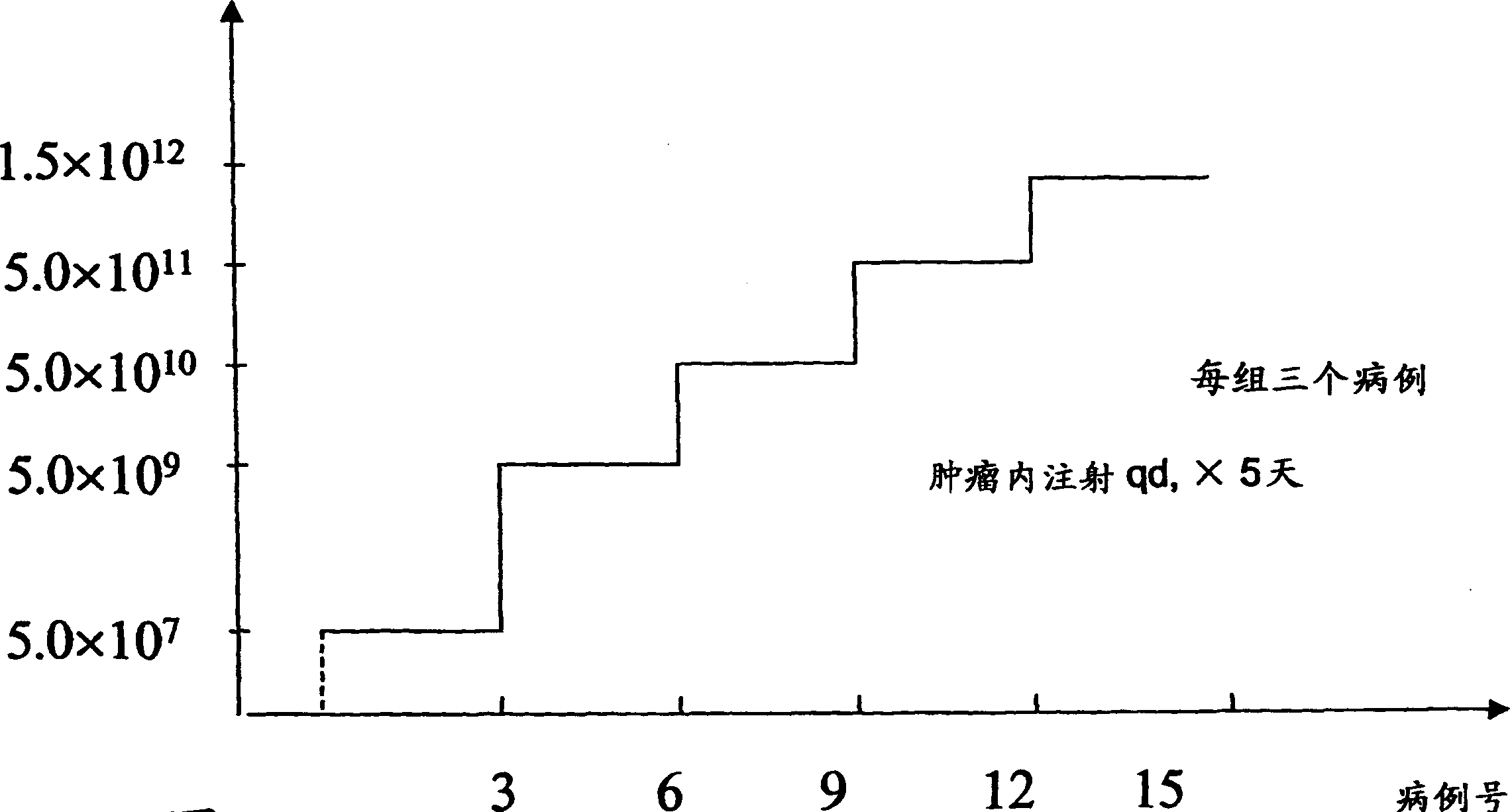
[0099] In order to determine an effective dosage regimen for animals (e.g., including humans) treated with the compositions and methods of the present invention, 5 dosage levels of H101 (SEQID#1) are used. Recombinant adenovirus is administered to patients with advanced solid tumors by intratumor injection. One goal is to determine the maximum drug resistance ("MTD") and the safety of intratumoral injection of H101 (SEQID#1). The 5 levels of H101 (SEQID#1) used are shown in Table 2, and the dose increase curve is shown in Figure 3. Each of the 5 independent dose levels includes 3 patients. The dose of DLT in 2 patients was determined as MTD, where DLT included influenza-like grade 4 toxicity due to H101 (SEQID#1), local reaction grade 4 toxicity at the injection site of H101 (SEQID#1), or due to Any toxicity of other grade 3 severity produced by H101 (SEQID#1). If one of three patients develops DLT, that group will treat a total of 6 patients.
[0100] Level
H101 (vi...
Embodiment 3
[0104] Use oncolytic virus S98-001 (SEQ ID#1) synchronized with high temperature to treat cancer patients. The patient’s date of birth was June 10, 1943. He was diagnosed with nasopharyngeal carcinoma in 1990. After a period of treatment with radiotherapy, the development of the primary tumor is clinically controlled. However, during these years, two tumors in the right neck and supraclavicular area slowly developed. At the end of 2001, these two tumors were treated with radiotherapy (cobalt-60, DT 34 Gy / 17F / 24d) combined with high temperature. Unfortunately, these treatments did not inhibit the progression of the two tumors. The patient was hospitalized at the beginning of February 2002. Before being treated with the oncolytic virus S98-001 (SEQID#1) synchronized with the high temperature, the patient underwent a physical examination. The patient is generally in good physical condition, but his nasopharyngeal tissue is nodularly thickened and slightly congested. The surfaces of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com