Liver protecting prepn containing silybin and total arasaponin and its prepn process and quality control method
A technology of silibinin and Panax notoginseng total saponins, which is applied to liver protection preparations containing silybin and Panax notoginseng total glucosides and the fields of their preparation and quality control, can solve the problem of limited curative effect, no compatibility of Panax notoginseng and silymarin Research reports, drug shortages, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The preparation of embodiment one liver-protecting injection
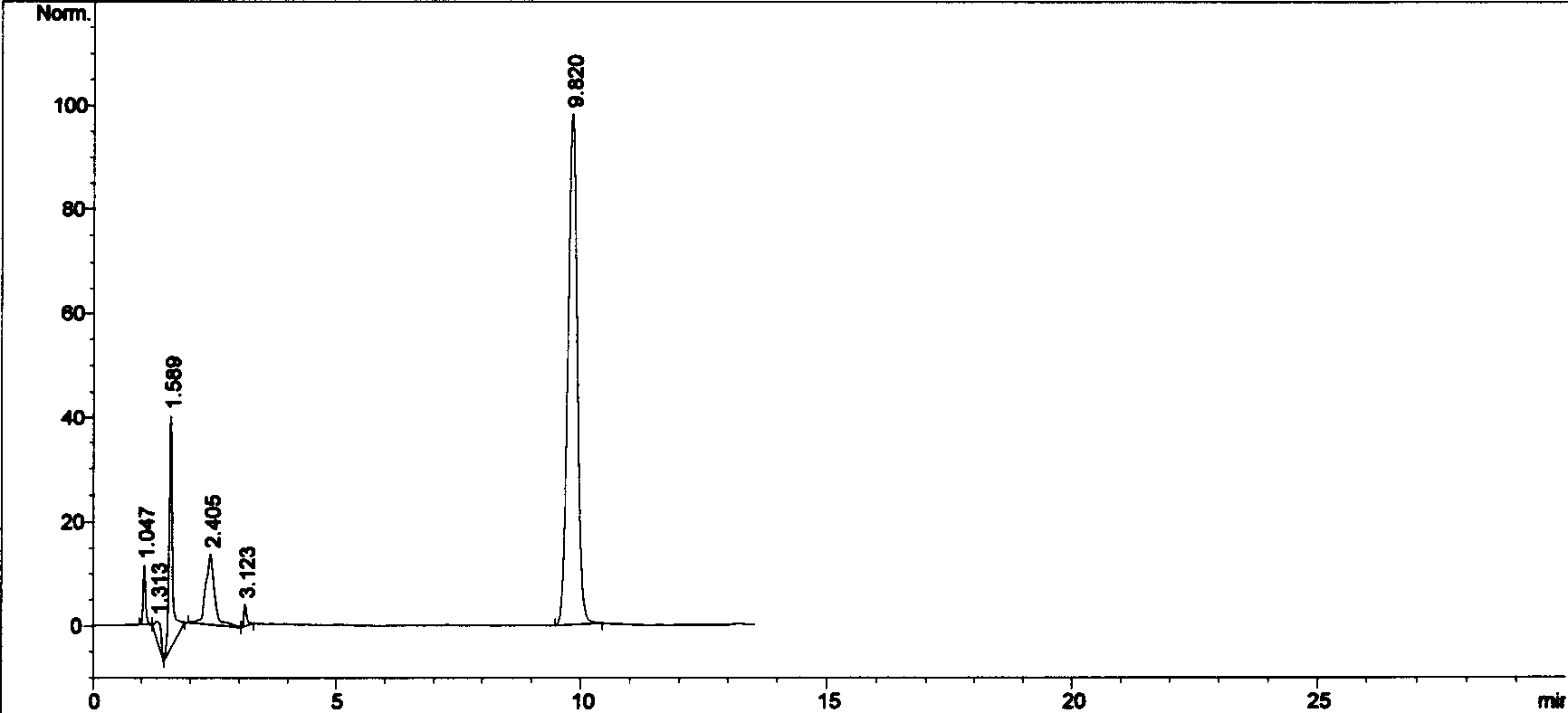
[0082] Take 100g of silibinin meglumine, add 5L of water for injection, stir, dissolve, centrifuge, add 100g of Panax notoginseng saponins to the supernatant, and then add 3L of co-solvent, which is either polyethylene glycol or propylene glycol , dissolved, diluted to 20L with an appropriate amount of water for injection, mixed evenly, filtered, the filtrate was ultrafiltered through an ultrafiltration membrane with a flux of 50,000 molecular weight, potted, 10ml / branch, and the injection was obtained.
Embodiment 2
[0083] The preparation of embodiment two compound medicine freeze-dried powder injection
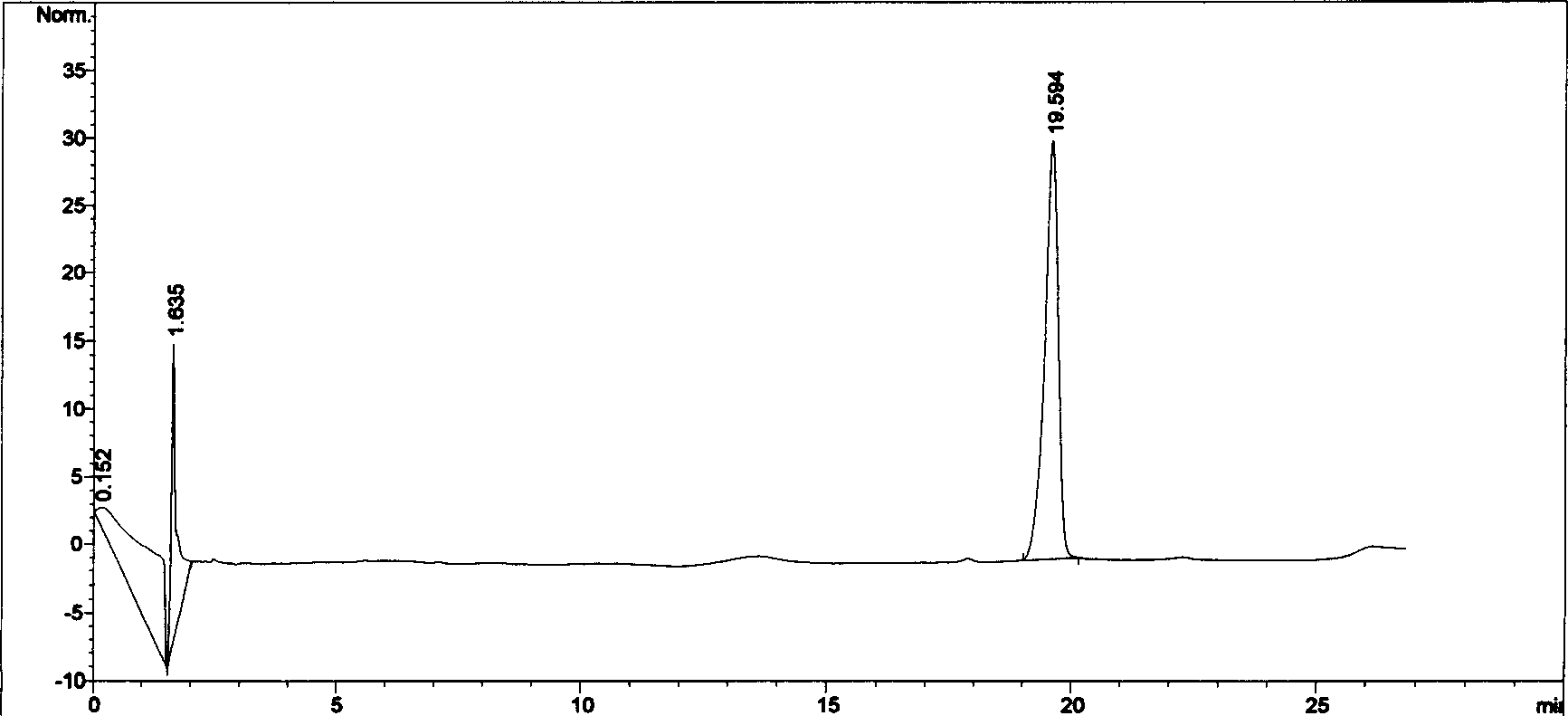
[0084] Take 125g of silybin meglumine, add 4L of water for injection, stir, dissolve, centrifuge, add 125g of Panax notoginseng saponins and 750g of molding agent to the supernatant, the molding agent is at least one of mannitol or glucose, and dissolve , diluted to 10L with an appropriate amount of water for injection, mixed evenly, filtered, the filtrate solution was ultrafiltered by an ultrafiltration membrane with a flux of 100,000 molecular weight, aseptically dried, divided into 2500 bottles, 4ml per bottle, freeze-dried, and capped , label, you can get the powder needle.
Embodiment 3
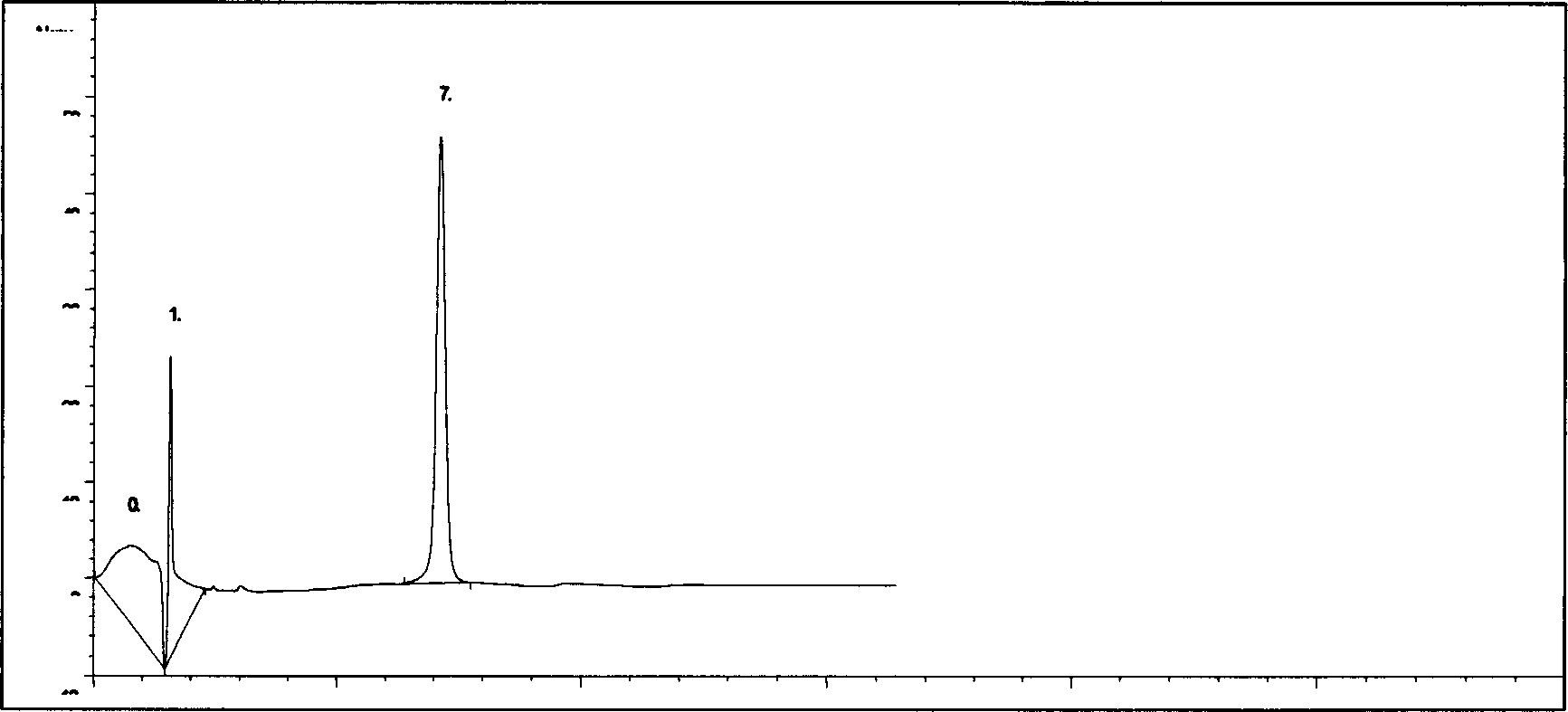
[0085] The rabbit erythrocyte hemolysis test comparison of embodiment three compound drug lyophilized powder and Panax notoginseng saponins solution
[0086] Referring to the pharmacological experiment methodology, take 20ml of blood from the heart of a rabbit, put it in a clean and dry beaker, stir it with a bamboo stick to remove fibrin, then transfer the blood into a centrifuge tube, add normal saline, mix well and centrifuge for 5 minutes (2500r / min) , discard the supernatant, add normal saline, mix and centrifuge, wash repeatedly until the supernatant is colorless and transparent, and then dilute the obtained red blood cells into a 2% suspension with normal saline according to its volume. Take 12 test tubes and divide them into two groups, put the number 1-6 on the test tube rack, put the number 1-6 on the test tube rack, add normal saline 2.4, 2.3, 2.2, 2.1, 2.0, 2.5ml, add 2% blood cell suspension solution of 2.5ml to each tube, then add No. 1-5 total saponins solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com