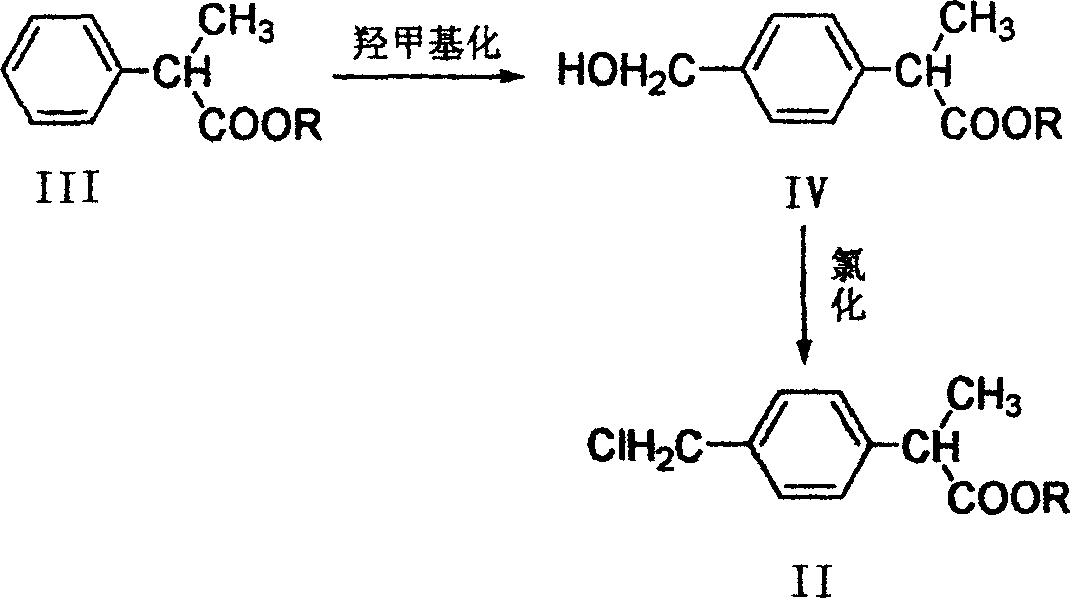
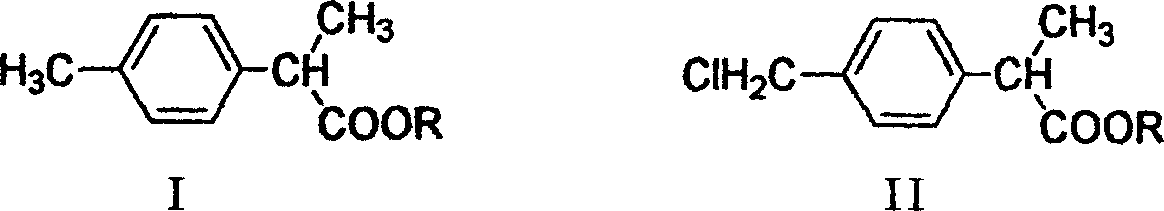Method for preparing 2-(P-chloromethyl phenyl) propionic acid/ester
A chloromethylphenyl, propionic acid technology, applied in the preparation of carboxylate, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of high cost, many steps, etc., and achieves low equipment requirements, simple operation, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 80g of chlorobenzene, 50g (0.305mol) of 2-(p-methylphenyl)propionic acid, and 2,2′-azobisisobutyronitrile to a 250ml round bottom flask equipped with a mechanical stirrer and reflux condenser. (AIBN) 2.46g (0.015mol), then control the reaction temperature to 70°C, slowly add 45.4g (0.336mol) of sulfonyl chloride dropwise, and complete the addition within 3h. The reaction mixture was cooled to room temperature, and then continued to be cooled to 0~5°C, and filtered with suction. The solid was recrystallized with a mixed solvent of benzene and petroleum ether to obtain 44.2 g of 2-(p-chloromethylphenyl)propionic acid with a yield of 73. %, the product is white crystals, and its purity is 98% as detected by high pressure liquid chromatography. 1H NMR (500M, CDCl3, ppm): 1.5 (3H, d, J = 7.0 Hz), 3.7 (1H, q, J = 7.0 Hz), 4.7 (2H, s), 7.3 (4H, dd), 11.3 ( 1H, s, br). IR (film): 3400, 2950, 1700, 1420, 1220 cm-1. ESI-MS: m / z(%)=199(100) [M+H].
Embodiment 2
[0026] Add 80g of chlorobenzene and 50g (0.305mol) of 2-(p-methylphenyl)propionic acid to a 250ml round-bottomed flask equipped with a mechanical stirrer and reflux condenser. Under the mercury lamp irradiation, the reaction temperature is controlled to 48 At ℃, 45.4g (0.336mol) of sulfonyl chloride was slowly added dropwise, and the addition was completed within 3h. The reaction mixture was cooled to room temperature, then continued to be cooled to 0~5°C, and filtered with suction. The solid was recrystallized with a mixed solvent of benzene and petroleum ether to obtain 43.0g of 2-(p-chloromethylphenyl)propionic acid with a yield of 71 %.
Embodiment 3
[0028] In a 250ml round bottom flask equipped with a mechanical stirrer and reflux condenser, 80g of benzene, 50g (0.305mol) of 2-(p-methylphenyl)propionic acid, and 3.63g (0.015) of benzoyl peroxide (BPO) were added. mol), and then control the reaction temperature to 75°C, slowly add 45.4g (0.336mol) of sulfonyl chloride dropwise, and complete the addition within 3h. The reaction mixture was cooled to room temperature, then continued to be cooled to 0~5°C, filtered with suction, and the solid was recrystallized with a mixed solvent of benzene and petroleum ether to obtain 42.4 g of 2-(p-chloromethylphenyl)propionic acid, with a yield of 70 %, the product is white crystals, and its purity is 98% as detected by high pressure liquid chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com