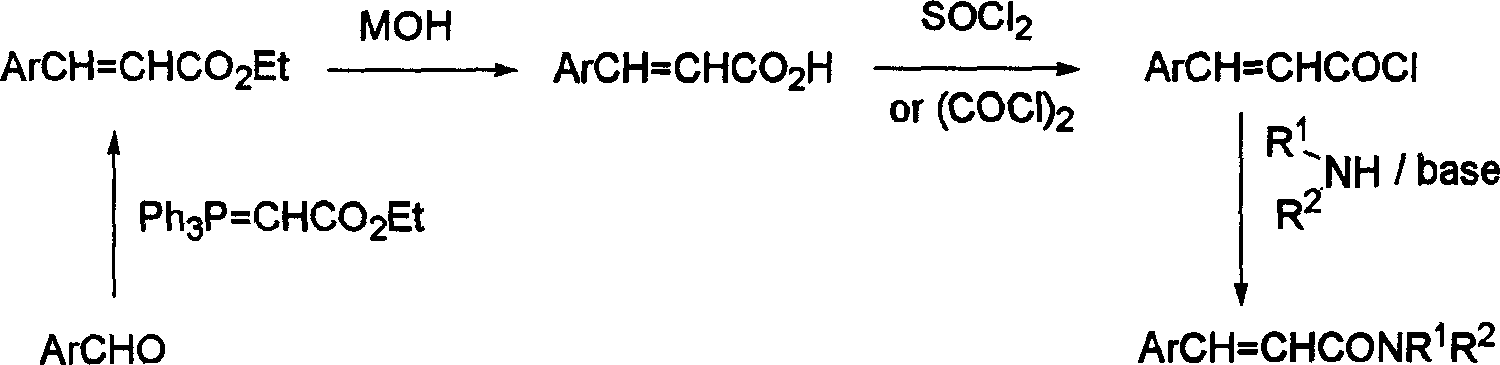Cinnamide compound synthesizing process
A technology for cinnamamide and compound, which is applied in the field of synthesizing cinnamamide compounds, can solve the problems of low synthesis efficiency, large environmental pollution, large energy consumption and the like, and achieves the effects of saving time, reducing reaction procedures and saving energy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Suspend 104.7 mg (0.54 mmol, 1.5 equivalents) of N-n-butyl α-bromoacetamide, 37 mg (0.35 mmol, 1.0 equivalents) of benzaldehyde, and 142 mg (0.54 mmol, 1.5 equivalents) of triphenylphosphine in a solution containing Lithium hydroxide 26 milligrams (0.65mmol, 1.8 equivalent) and lithium chloride water (5ml) solution of 1.2 molar concentration, reflux reaction 10 minutes, reaction solution is cooled to room temperature then, extract with ethyl acetate (10ml * 3) , the combined organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to obtain a crude product. The crude product was separated and purified by silica gel column chromatography (petroleum ether: ethyl acetate = 5: 1) to obtain a yellow oil, 69 mg of N-n-butyl cinnamic acid, the yield was 96%, and the E / Z ratio was 86 / 14.
[0041] (E-isomer) 1 H NMR (400MHz, CDCl 3 )δ7.61(d, J=15.6Hz, 1H), 7.48-7.46(m, 2H), 7.34-7.30(m, 3H), 6.45(d...
Embodiment 2
[0043] 194 mg (1.00 mmol, 1.8 equivalents) of N-n-butyl α-bromoacetamide and 78 mg (0.56 mmol, 1.0 equivalents) of 4-chlorobenzaldehyde, 262 mg (1.00 mmol, 1.8 equivalents) of triphenylphosphine Suspended in lithium hydroxide 52 mg (1.23 mmol, 2.1 equivalents) and 1.2 molar lithium chloride water (5 ml) solution, refluxed for 10 minutes. Aftertreatment is with embodiment 1. The crude product was separated and purified by silica gel column chromatography (petroleum ether: ethyl acetate = 3: 1) to obtain a white solid (mp.118-120°C), 132 mg of N-n-butyl-3-phenylacrylamide, and the yield was 100% with an E / Z ratio of 86 / 14.
[0044] (E-isomer) 1 H NMR (400MHz, CDCl 3 )δ7.57(d, J=16.0Hz, 1H), 7.41(d, J=7.6Hz, 2H), 7.32(d, J=7.6Hz, 2H), 6.37(d, J=16.0Hz, 1H) , 5.77(bs, 1H), 3.41-3.36(m, 2H), 1.59-1.51(m, 2H), 1.43-1.34(m, 2H), 0.96-0.92(t, J=7.2Hz, 3H).( Z-isomer) 1 HNMR (400MHz, CDCl 3 )δ7.41(d, J=8.4Hz, 2H), 7.29(d, J=8.4Hz, 2H), 6.65(d, J=12.4Hz, 1H), 5.97(d, J=12.4Hz, 1H...
Embodiment 3
[0046] 194 mg (1.00 mmol, 1.8 equivalents) of N-n-butyl α-bromoacetamide and 84 mg (0.56 mmol, 1.0 equivalents) of 4-nitrobenzaldehyde, 262 mg (1.00 mmol, 1.8 equivalents) of triphenylphosphine ) was suspended in lithium chloride water (5ml) solution containing lithium hydroxide 52 mg (1.23mmol, 2.1 equivalents) and 1.2 molar concentration, and refluxed for 5 minutes. Aftertreatment is with embodiment 1. The crude product was separated and purified by silica gel column chromatography (petroleum ether: ethyl acetate = 3:1) to obtain a yellow solid (mp.126-128°C), N-n-butyl-3-(4-nitrophenyl)acrylamide 112 mg, 80.5% yield, E / Z ratio 95 / 5.
[0047] (E-isomer) 1 H NMR (400MHz, CDCl 3 )δ8.19(d, J=8.4Hz, 2H), 7.65(d, J=15.6Hz, 1H), 7.63(d, J=8.4Hz, 1H), 6.55(d, J=15.6Hz, 1H) , 5.98(bs, 1H), 3.42-3.37(m, 2H), 1.58-1.52(m, 2H), 1.41-1.36(m, 2H), 0.96-0.91(t, J=7.2Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com