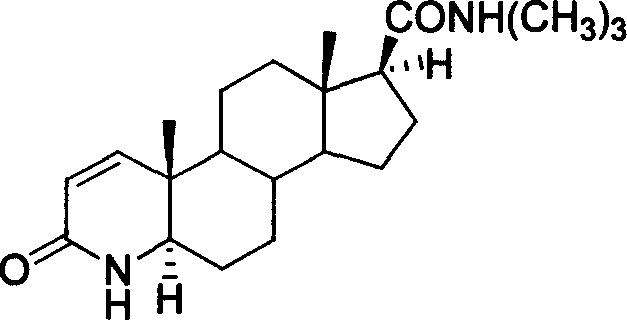
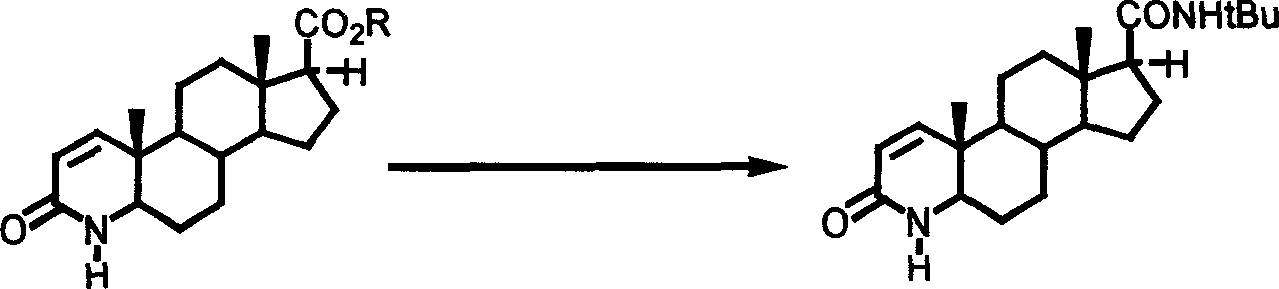
Synthesis method of N-tertiary butyl-3-carbonyl-4-aza-5 alpha-androl-1-end-17 beta-formamide
A technology of tert-butyl and formamide, which is applied in the field of synthesizing the steroid drug finasteride, can solve the problems of lack of novelty, many reaction steps, expensive sulfide, etc., and achieve convenient crystallization and purification, fewer reaction steps, and cheap raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
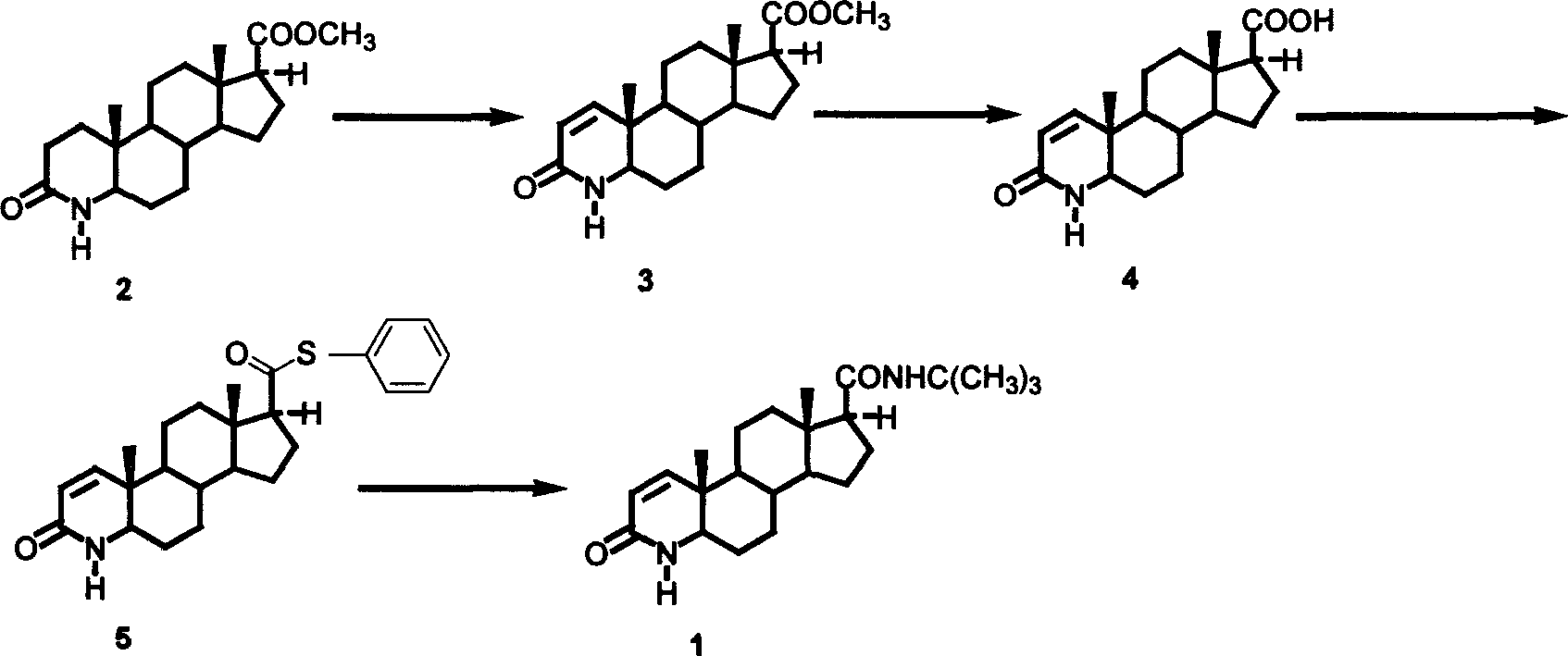
Embodiment 1
[0031] 1. 5,17-dicarbonyl-3,5-cyclo-androst-3-acid (3)
[0032] 4-androstene-3,17-dione (2) 4.0g, tert-butanol 100ml, dropwise add potassium permanganate 0.54g, anhydrous potassium carbonate 4.0g, sodium periodate 16.9g at 50-60°C The aqueous solution was added, and the reaction was maintained at 50-60°C for 3h. Cool to room temperature and filter off the solid. Most of the tert-butanol was evaporated from the filtrate under reduced pressure, and the residual liquid was adjusted to about pH 2 with 6 mol / L hydrochloric acid in an ice bath. Extracted with dichloromethane, dried over anhydrous sodium sulfate, and evaporated to dryness to give white solid 3 (3.8 g, 91.2%), IR (KBr): 3050-3500, 1735, 1702.
[0033] 2. 4-aza-5-androstene-3,17-dione (4)
[0034] 3.8g of the above product (3), 2.9g of ammonium acetate, 40ml of glacial acetic acid, stirred and heated to reflux for 4h, evaporated to remove the acetic acid, poured the residue into water, washed the precipitate with su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com