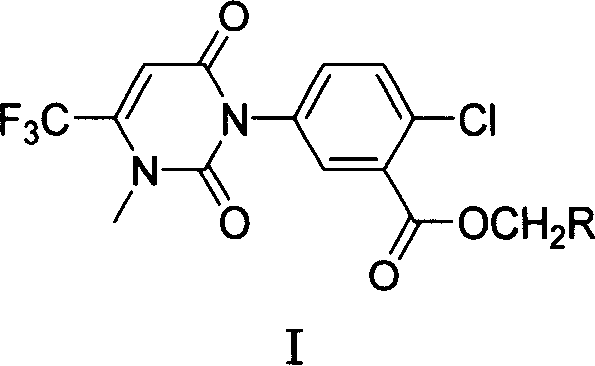
Compound of 1-pyrimidine ketone group-4-chlorine-5-benzoic ethers and preparation method thereof
A technology of ester compound and pyrimidinone group, which is applied in the field of 1-pyrimidinone-4-chloro-5-benzoic acid ester compound and its preparation, can solve the problem of 1-pyrimidinone-4-chloro-5- 5-Benzoic acid monosubstituted methyl ester compounds and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
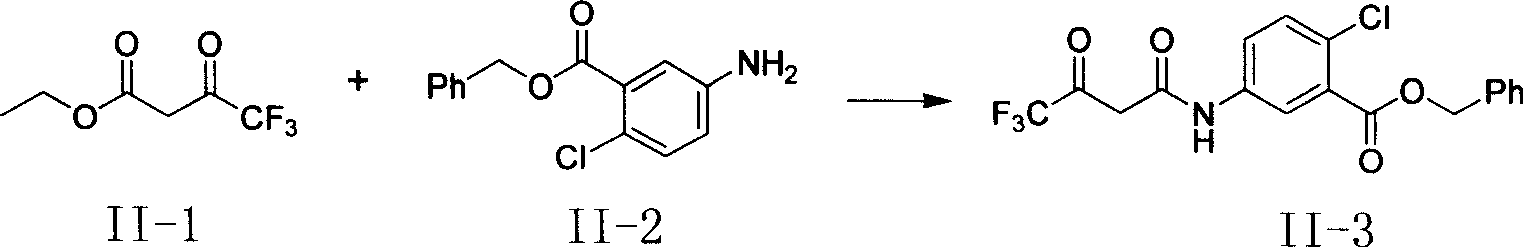
[0033] Synthesis of Compounds (II-3)~(II-7)
[0034]
[0035] Step 1: Add 30mmol of compound (II-2), 30-36mmol of ethyl trifluoroacetoacetate (II-1) and 250mL of toluene into a 500mL three-neck flask, install a stirring and distillation device, stir and heat slowly at 10°C At the same time, the toluene in the reaction solution and the ethanol generated were distilled out, an average of 20 mL per hour; after the reaction was carried out for 1 hour, 3 mmol of ethyl trifluoroacetoacetate (II-1) was added to continue the reaction, and then added after 2 hours Ethyl trifluoroacetoacetate (II-1) 3mmol and toluene 40mL, stop the reaction after 4 hours. The reaction solution was concentrated and purified by silica gel column chromatography, the developing solvent was ethyl acetate / n-hexane=1 / 5 to obtain 15 mmol of waxy solid (II-3) with a yield of 50%. 1 H NMR (CDCl 3 , 300MHz): δ5.260(s, 1H), 5.346(s, 2H), 7.425(m, 6H), 7.747(m, 1H), 7.898(s, 1H).
[0036] Step 2: Add (II-3) 15...
Embodiment 2
[0041] R=CH in the compound general formula (I) of the present invention listed in table 1 3 -Synthesis:
[0042]
[0043] 0.5 mmol of compound (II-7) prepared in Example 1, 1 mmol of ethyl iodide, 0.6 mmol of potassium carbonate and 10 mL of DMF were added to a 20 mL three-necked flask and stirred at 65° C. for 3 hours. The residue was poured into 10 ml of water, extracted twice with ethyl acetate, 20 ml each time, washed with 15 ml of saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, and the residue was passed through a silica gel column. Purified by chromatography, the eluent was ethyl acetate / n-hexane=1 / 3, and the compound (I)-1 in Table 1 was obtained as a white solid 0.33 mmol, the yield was 66%, and the melting point was 101-102°C. Replace the solvent DMF with chloroform, dichloromethane, carbon tetrachloride, hexane, benzene, toluene, ethyl acetate, acetone, THF or dioxane, and replace the base ...
Embodiment 3
[0045] The compounds of the present invention listed in Table 1 have the general formula (I) formula R=CH 3 CH 2 -Synthesis:
[0046] Except that iodoethane was replaced by iodopropane, other operating procedures were the same as in Example 2, and compound (I)-2 in Table 1 was obtained as a white solid, melting point: 98-99°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com