Nuclear factor-K B P65 subunit antagonistic peptide and its use
A nuclear factor and antagonistic peptide technology, applied in the field of medicine, can solve difficult-to-reach problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
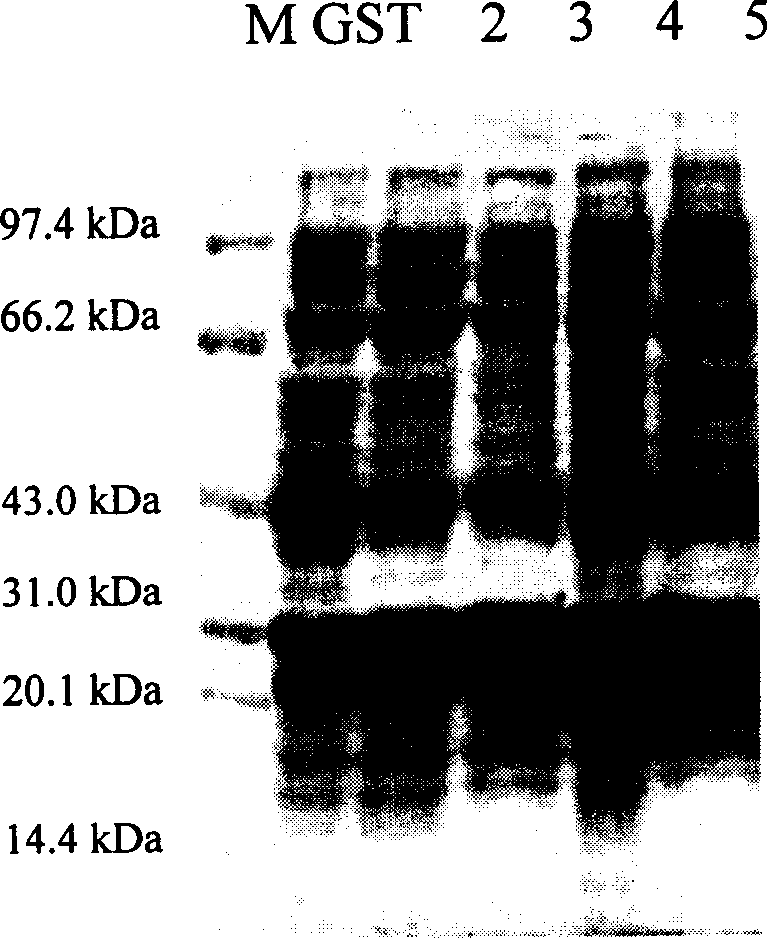
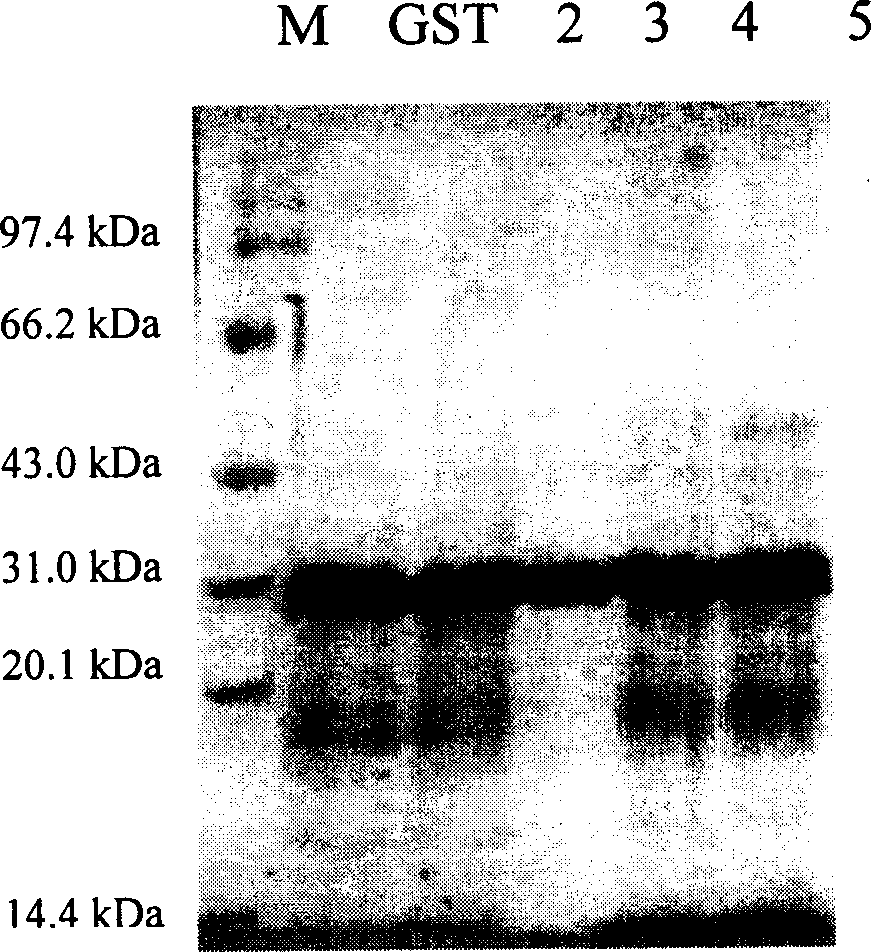
[0119] Example 1, GST fusion expression and purification of nuclear factor-κB p65 subunit antagonistic peptide
[0120] According to the gene sequences of PT1, PT2, PT3, and PT4 polypeptides, while keeping the polypeptide protein coding sequence unchanged, according to the codon preference of Escherichia coli, respectively design and synthesize complementary DNAs with BamHI in the upstream sequence and SalI cohesive ends in the downstream sequence Fragment, the DNA sequence is as follows:
[0121] PT1-FP: 5'-GATCGTTGTAATGATCGAAGTAGTTTTTCCTGTAG-3',
[0122] PT1-RP: 5'-TCGACTACAGGAAAACTACTTCGATCATTACAAC-3'
[0123] PT2-FP:
[0124] 5'-GATCCTGGCGATGGTTGAAGTAACTGTTGTTCTGTCTTGGGGTTTCACTTAG-3'
[0125] PT2-RP: 5'-
[0126] TCGACTAAGTGAAACCCCCAAGACAGAACAACAGTTACTTCAACCATCGCCAG-3’
[0127] PT3-FP:
[0128] 5′-GATCCCGGCGATGGTTGAAGTAACTGTTGTTCTGTCTTGGGGTTTCACTTAG-3′
[0129] PT3-RP:
[0130] 5′-TCGACTAAGTGAAACCCCAAGACAGAACAACAGTTACTTCAACCATCGCCGG-3′
[0131] PT4-FP:
[0132]...
Embodiment 2
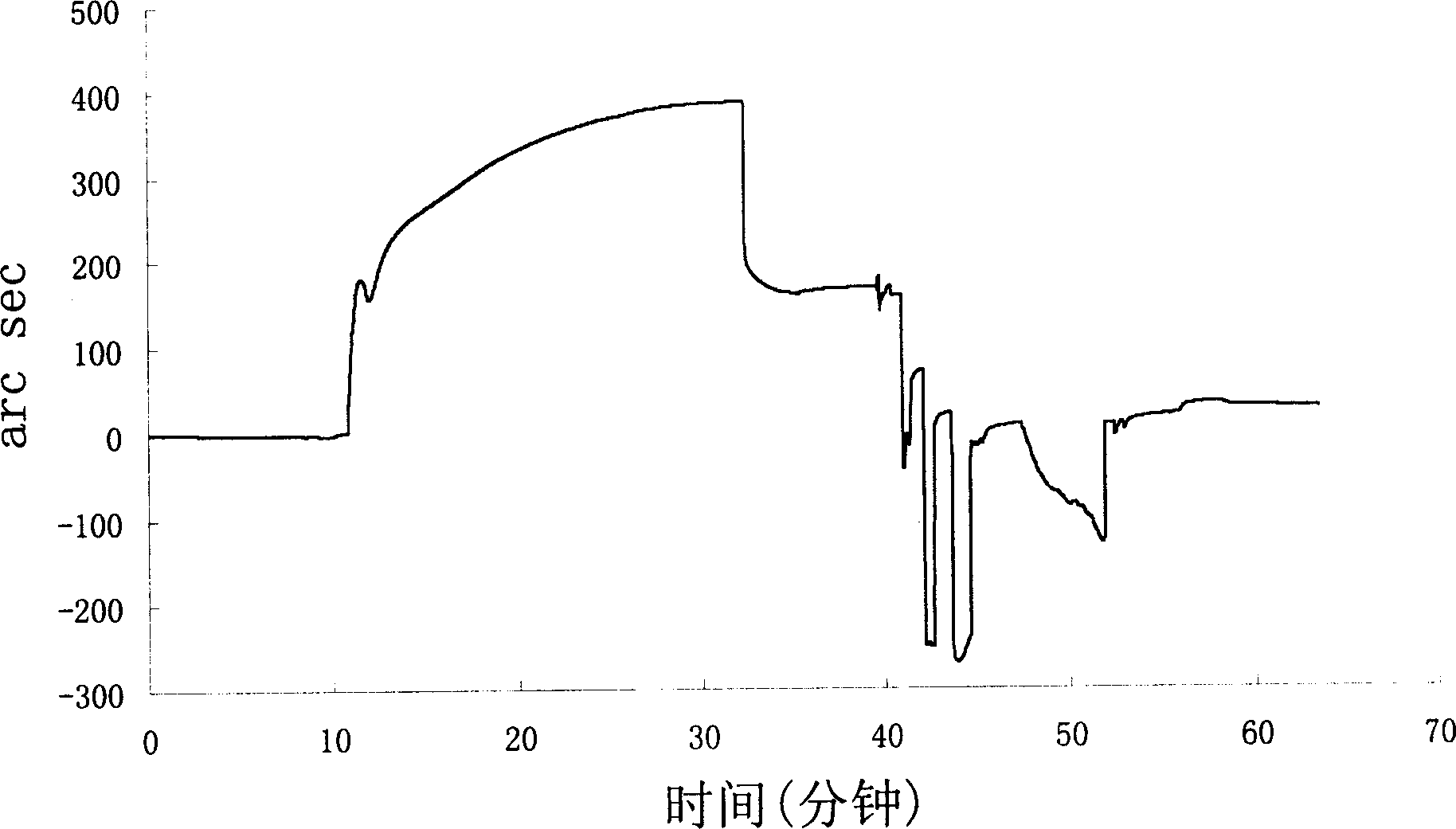
[0137] Example 2, Biosensor (Biosensor) detects the combination of GST fusion peptide and nuclear factor-κB p65 subunit
[0138] Put the carboxymethyl dextran biosensor chip into the microfluidic chuck of the Iasys Plus system, rinse with 60 μl PBS / T (pH 7.4PBS, 0.05% Tween 20) for 3 times, equilibrate for 10min, and collect after the baseline leveled off Baseline data for 5 minutes; mix EDC and NHS at a ratio of 1:1, take 40 μl of the mixed solution to wash the sample pool twice, then add 40 μl of the mixed solution to the sample pool, and keep it for 7 minutes to activate the surface of the sensor sheet; use 50 μl of PBS / T Rinse the sample pool 3 times to collect the baseline value for 1 min; wash the sample pool with 40 μl of 10 mM pH5.5 acetate buffer solution for 3 times, then add 40 μl of acetate buffer solution, and collect the baseline value for 1 min; then add 40 μl of acetate buffer solution to the sample pool The total amount of dilution is about 0.5 μg of p65 sta...
Embodiment 3
[0141] Example 3, ELISA method to detect the specific binding of GST fusion peptide and nuclear factor-κB p65 subunit
[0142] Wash the coated wells of the ELISA plate with deionized water several times, invert the plate, and tap the plate on a clean filter paper to remove the remaining water; dilute the p65 protein with PBS buffer at a concentration of 10 μg / ml; add 100 μl of the diluted p65 protein Put it into the coated wells of 96-well ELISA plate, incubate at room temperature for 3-4 hours or overnight at 4°C, pay attention to the evaporation of water; wash the wells with 300 μl PBS three times to remove unbound p65 protein; prepare 5% aqueous solution of skimmed milk powder , add 200μl per well, incubate at room temperature for 1h or incubate overnight at 4°C to seal the coated wells; use protein binding buffer (50mM Tris-HCl, pH7.2, 100mM NaCl, 10mM MgCl 2 , 10uM ZncL 2 , 1mM DTT, 0.1% (v / v) NP40) to wash the plate 5 times; add 0.5μmol / L GST fusion peptide (diluted i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com