Sustained releasing microspheric preparation of glucokinase mutant and its making method
A sustained-release microsphere preparation and technology of sustained-release microspheres, which are applied in the field of biopharmaceuticals, can solve the problems that there is no research report on staphylokinase mutant sustained-release microspheres, and achieve uniform appearance, good biocompatibility, and encapsulation. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
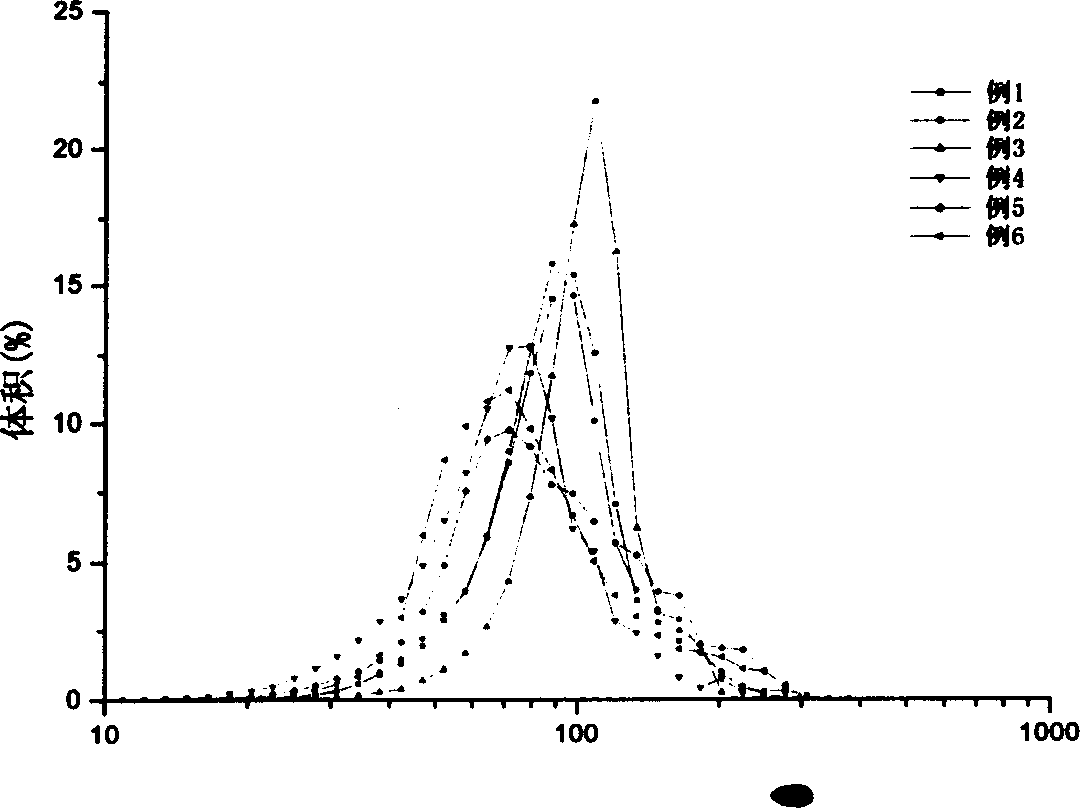
[0037]Emulsify 0.2ml of 10mg / ml DGR solution (0.02M phosphate buffer, pH 7.4) into 2.5ml of PLGA in dichloromethane solution, and ultrasonically emulsify for 30s at a power of 80W to obtain a W / O emulsion . Inject the emulsion into 75ml of 0.02M phosphate buffer (pH7.4) containing 2% PVA with a micro sampler, stir at a speed of 600rpm for 1min to prepare a W / O / W emulsion, and pour the emulsion into 225ml of 0.02M phosphate buffer solution, stirred at room temperature at 300rpm for 6h, evaporated the organic solvent, centrifuged to collect the microspheres after solidification, washed three times with double distilled water, freeze-dried, and stored at low temperature.
[0038] The obtained microspheres have an average particle diameter of 95.1 μm, an encapsulation efficiency of 7.09%, and a drug loading capacity of 0.068%.
Embodiment 2
[0040] Emulsify 0.2ml of 10mg / ml DGR solution (0.02M phosphate buffer, pH 7.4) into 2.5ml of PLGA in dichloromethane solution, and ultrasonically emulsify for 30s at a power of 80W to obtain a W / O emulsion . Inject the emulsion into 75ml of 0.02M phosphate buffer (pH7.4) containing 2% PVA and 2.5% sodium chloride with a micro sampler, stir at a speed of 600rpm for 1min to prepare a W / O / W emulsion , Pour the emulsion into 225ml 0.02M phosphate buffer, stir at 300rpm at room temperature for 6h, evaporate the organic solvent, collect the microspheres by centrifugation after solidification, wash three times with double distilled water, freeze-dry, and store at low temperature.
[0041] The obtained microspheres have an average particle diameter of 93.7 μm, an encapsulation efficiency of 45.4%, and a drug loading capacity of 0.0.46%. The active DGR in the microspheres accounted for 75.0%, and the insoluble aggregates accounted for 25%.
Embodiment 3
[0043] 0.2ml of 10mg / ml DGR solution (0.02M phosphate buffer containing 2% PVA, pH 7.4) was emulsified in 2.5ml of PLGA in dichloromethane solution, and ultrasonically emulsified for 30s at a power of 80W. A W / O emulsion is obtained. Inject the emulsion into 75ml of 0.02M phosphate buffer (pH7.4) containing 2% PVA with a micro sampler, stir at a speed of 600rpm for 1min to prepare a W / O / W emulsion, and pour the emulsion into 225ml of 0.02M phosphate buffer solution, stirred at room temperature at 300rpm for 6h, evaporated the organic solvent, centrifuged to collect the microspheres after solidification, washed three times with double distilled water, freeze-dried, and stored at low temperature.
[0044] The obtained microspheres have an average particle diameter of 104.3 μm, an encapsulation efficiency of 12.5%, and a drug loading capacity of 0.119%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com