Novel therapeutic use of polypodium extracts
A technology of water keel and extract, which is applied to medical preparations containing active ingredients, medical raw materials derived from ferns/filamentous plants, drug combinations, etc., which can solve problems such as fever, discomfort, and reduction of patients' sexual desire
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
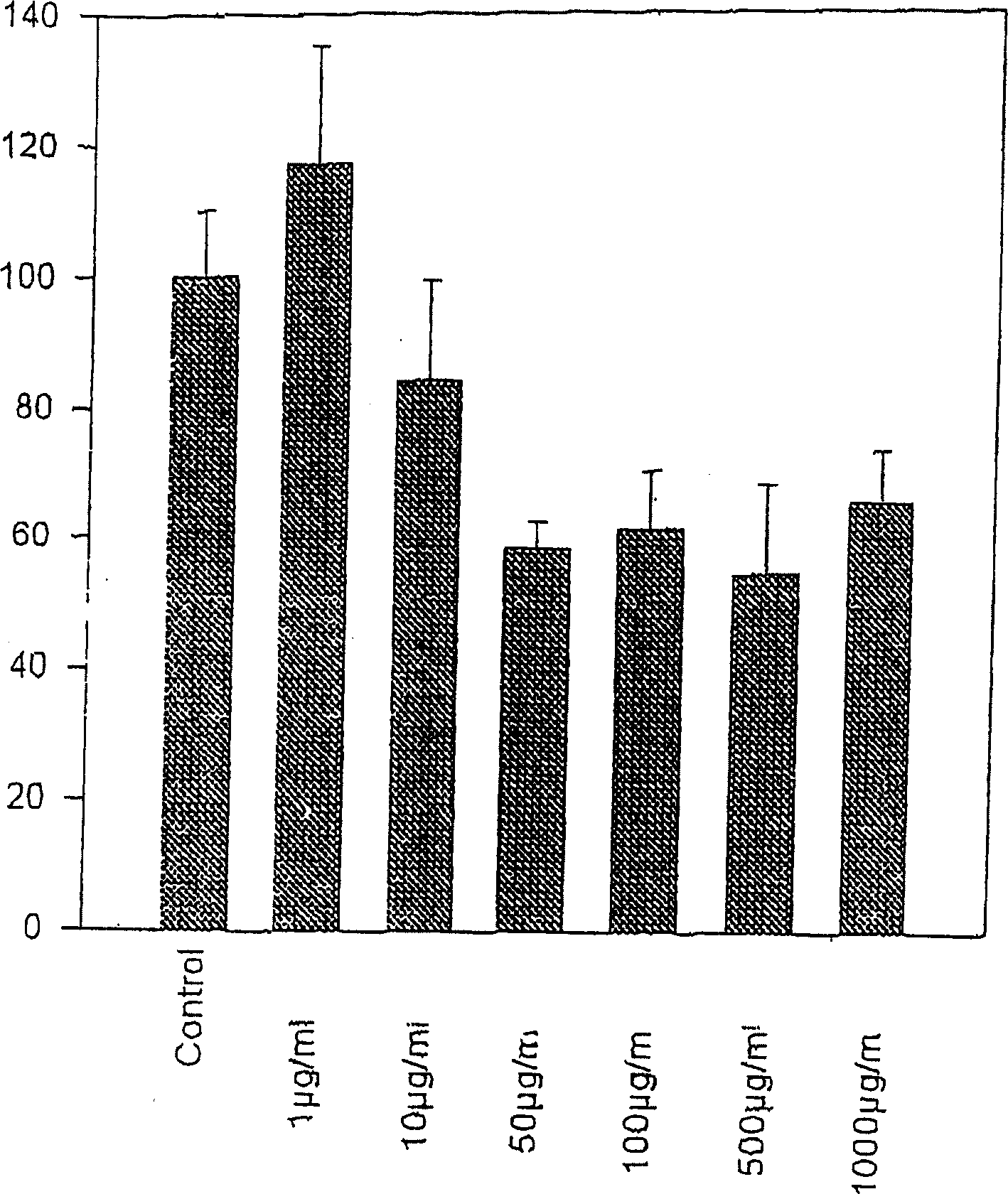
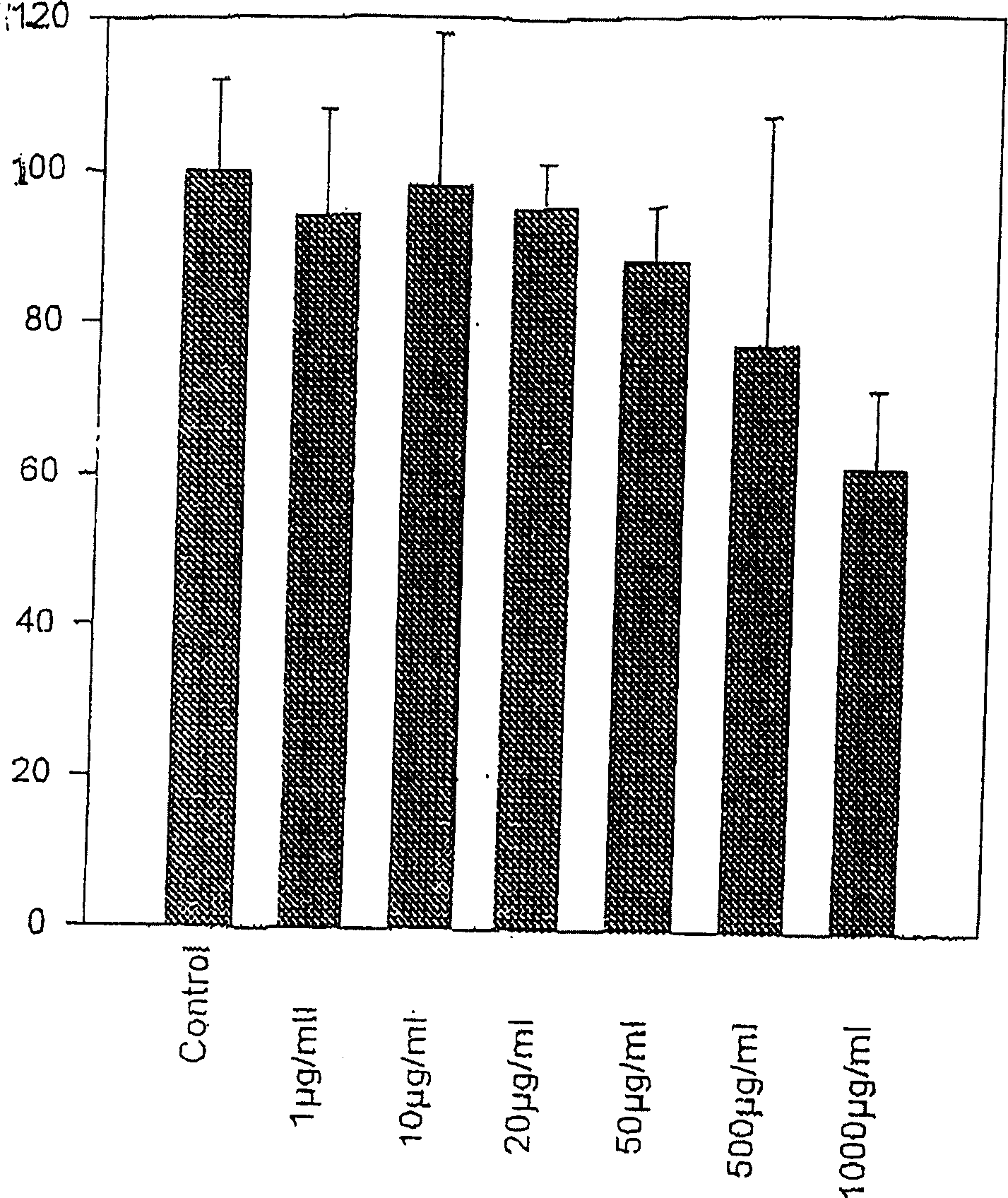
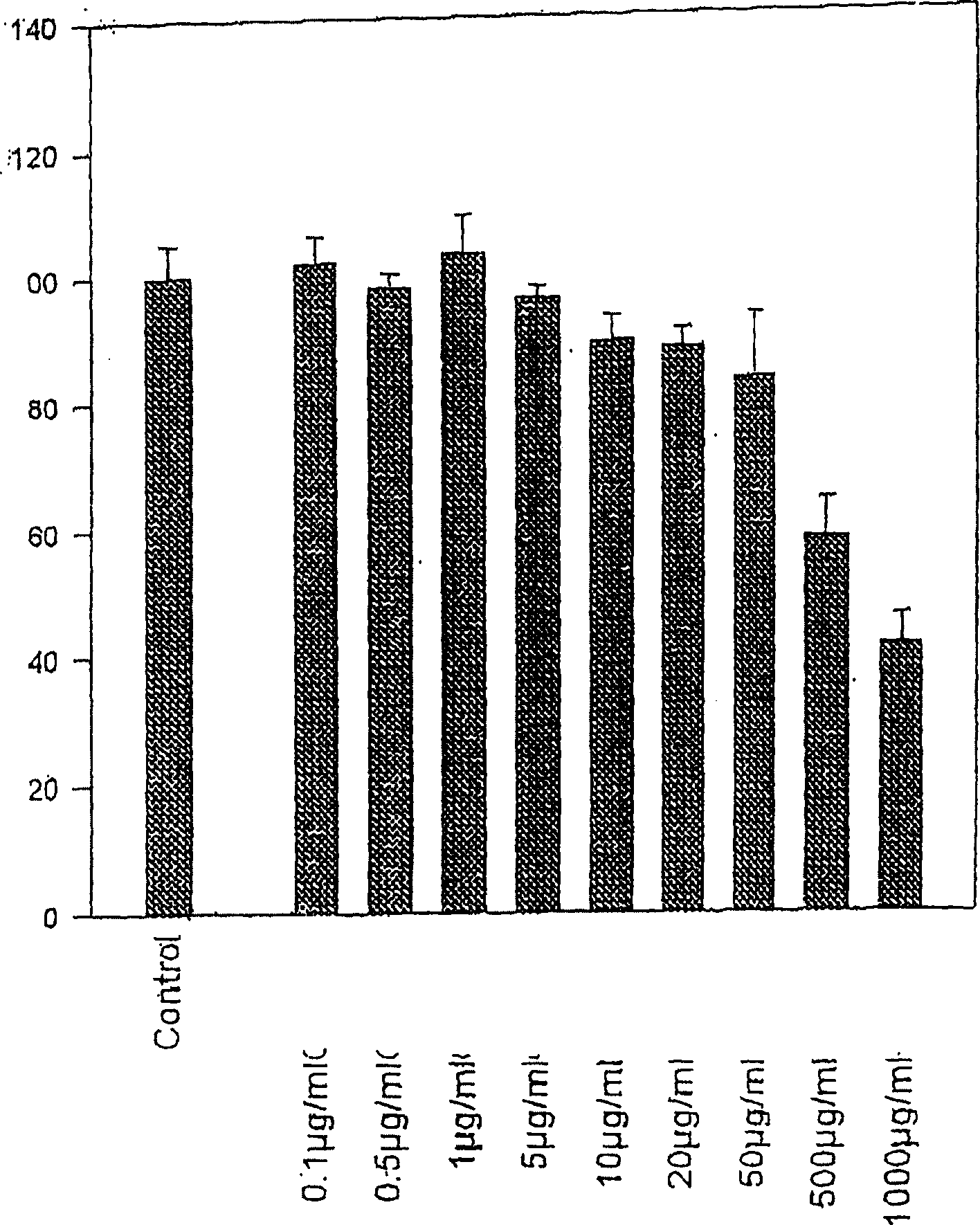
[0062] Example 1. Effect of Water Keelia Extract on Extracellular Matrix
[0063] Water keel extract
[0064] It was firstly extracted for 48 hours, then filtered with a membrane, and then the solvent was removed under low pressure, then dissolved in the physiological serum again, and finally freeze-dried to obtain the extract of the genus Thuisi. Extracts can be dissolved in PBS and used after regeneration.
[0065] cell culture
[0066] Obtain fibroblasts from surgical material. The skin fragments were placed in RPMI1640 medium at 4°C and 2% (double dose) penicillin and streptomycin solution, and pre-incubated for 2 hours. After removal of adipose tissue, small pieces of skin were fixed in microwells moistened with fetal calf serum (FCS). at 5% CO 2 Skin cells were cultured in RPMI1964 medium containing 10% FCS and 1% penicillin / streptomycin solution under gaseous environment. This medium is updated twice a week. Fibroblasts were first cultured in Tripsin and EDTA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com