Cancer-associated epitope
An epitope and adenocarcinoma technology, applied in the translation products of oncogenes, anti-receptors/cell surface antigens/cell surface determinant immunoglobulins, drug combinations, etc., can solve problems such as lack of filament elongation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0246] The preparation of monoclonal antibodies is also routine. For example, see Kohler & Milstein, Nature, 256: 495 (1975); Coligan et al., sections 2.5.1-2.6.7; and Harlow et al., in: Antibodies: A Laboratory Manual, page 726 (Cold Spring Harbor Pub. (1988) ), the above documents are included as references in this article. A variety of well-established techniques can be used to isolate and purify monoclonal antibodies from hybridoma cultures. The separation techniques include affinity chromatography with Protein A Sepharose, size exclusion chromatography, and ion exchange chromatography. For example, see Coligan et al., sections 2.7.1-2.7.12 and sections 2.9.1-2.9.3; Barnes et al., Purification of lmmunoglobulin G (IgG), in: Methods in Molecular Biology, Vol. 10, pages 79-104 ( Humana Press (1992).
[0247] Methods of manipulating monoclonal antibodies in vitro and in vivo are well known to those skilled in the art. For example, the monoclonal antibodies used in the present inv...
Embodiment 1
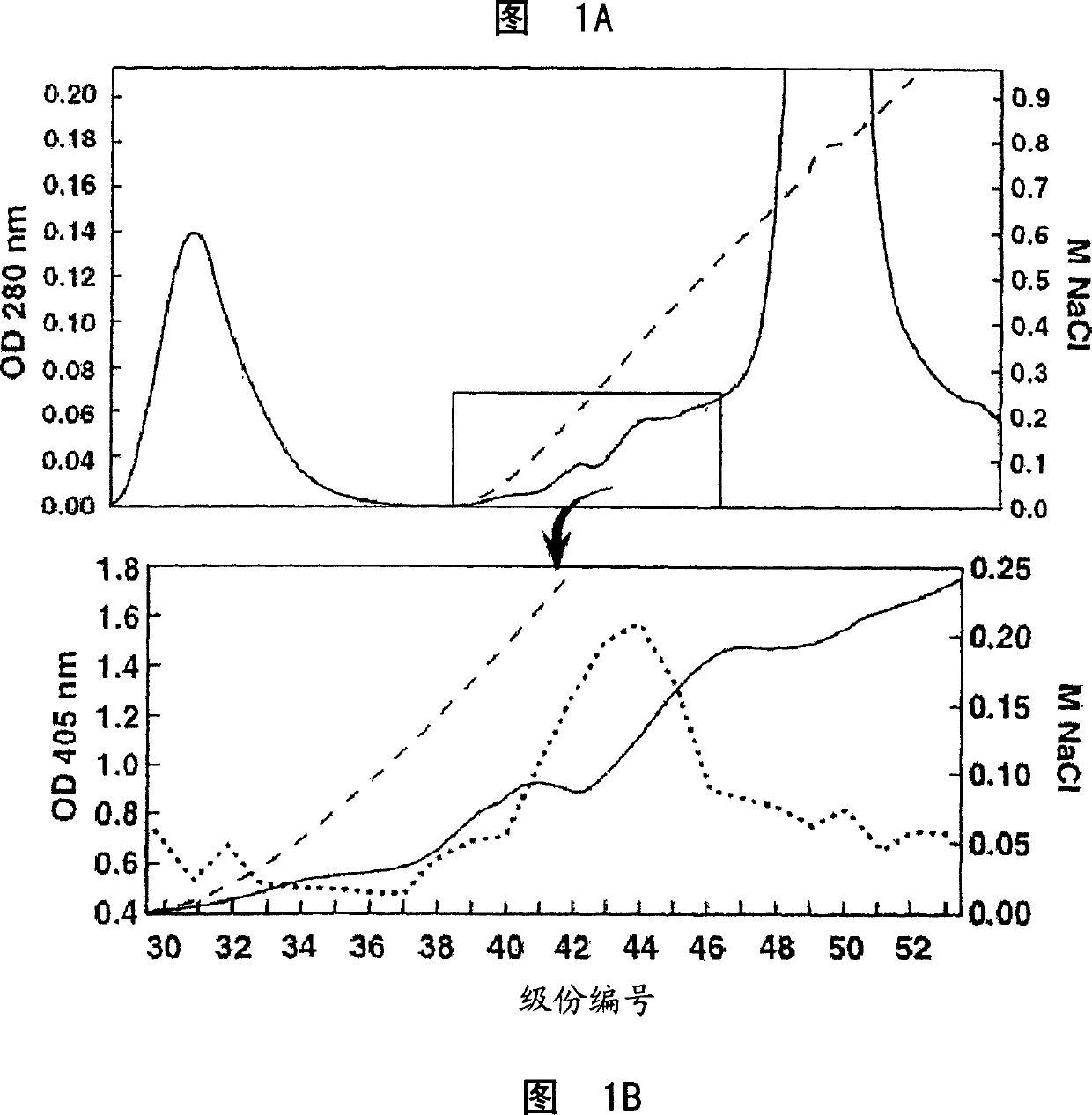
[0458] Example 1: Characterization of cancer-related epitopes
[0459] Separation of COU-1 monoclonal antibody
[0460] IgM HMab, COU-1 is secreted by the hybridoma cell line B9165, which is obtained by combining the human lymphoblastoid cell line WI-L2-729-HF2 with the lymph nodes obtained from the mesenteric lymph nodes of colon cancer patients. Produced by cell fusion (35). Under aseptic conditions, mince the mesenteric lymph nodes from the tumor site of patients with colorectal cancer. The debris was removed by filtration through cotton yarn, and the lymphocytes were purified by centrifugation on Ficoll-Isopaque (Boehringer-Mannheim, Old Federal Republic of Germany and Italy).
[0461] The lymphocytes were fused with the human fusion cell line W1-L2-729-HF2 (referred to as HF2) (from Tecniclone Int., Santa Ana, Calif., USA) according to the method disclosed in the following document: Kohler, Immunological Methods Vol. II, Academic Press, 1981, pp. 285-298. Th...
Embodiment 2
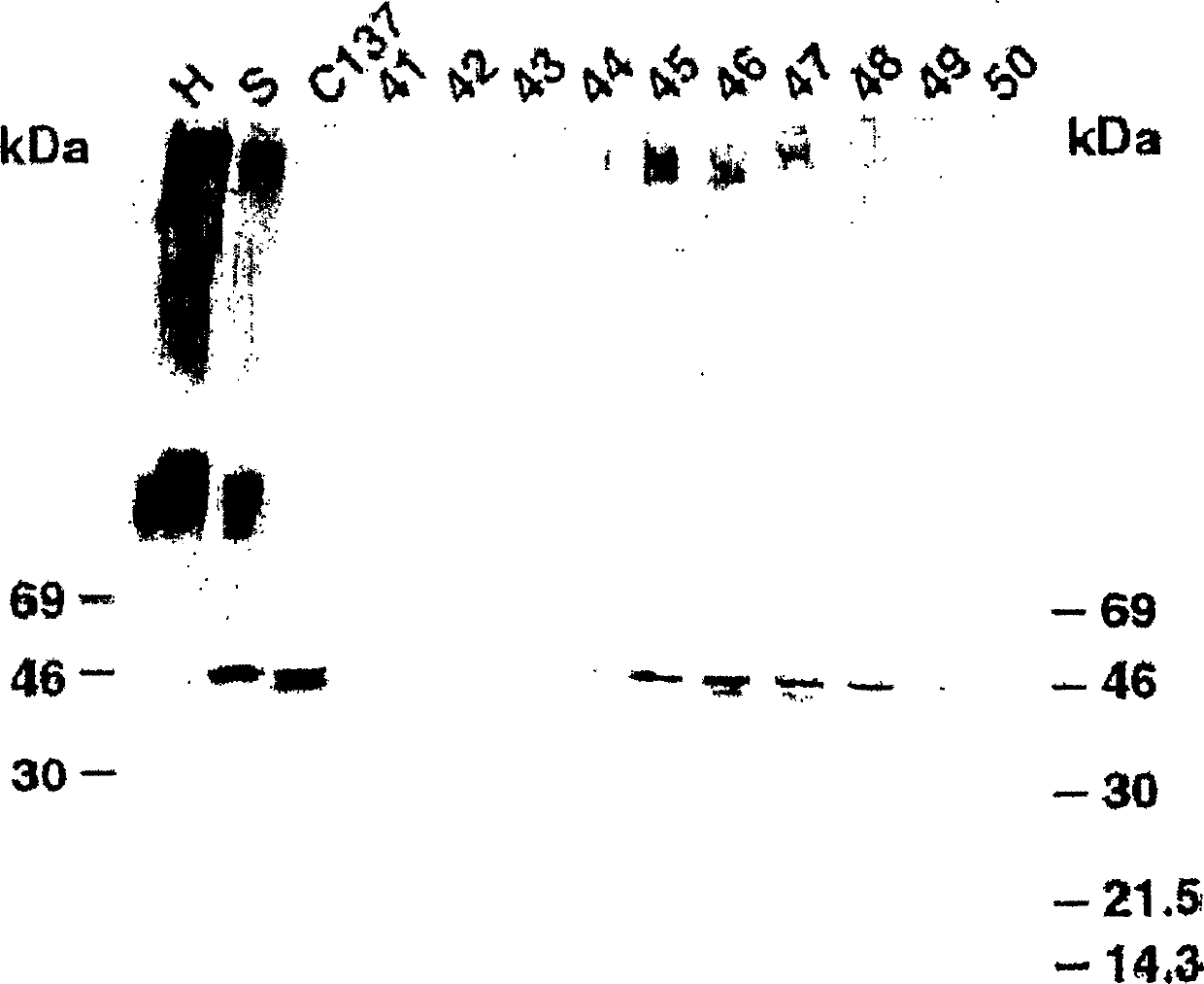
[0501] Example 2: Cancer-related epitope mapping
[0502] Materials and methods
[0503] antibody
[0504] As mentioned above, IgM HMab, COU-1, is secreted by the hybridoma cell line B9165, which is obtained by combining the human lymphoblastoid cell line WI-L2-729-HF2 with the mesentery of colon cancer patients. Produced by the fusion of lymphocytes obtained in a lymph node. The hybridoma cell line B9165 was handed over to the European Cell Culture Collection (ECACC), CAMR, Salisbury, Wiltshire, SP4 OJG, UK, with the deposit number ECACC 87040201. More information about ECACC can be found on the ecacc.org website.
[0505] The human-human hybridoma cell line was grown in protein-free medium: RPMI1640 medium (Gibco, Grand Island, NY) supplemented with SSR3 serum replacement (Medicult, Copenhagen, Denmark). HMab COU-1 is purified from cell culture supernatant by affinity chromatography on agarose-conjugated murine anti-human μ-chain monoclonal antibody (Mab) (HB57, ATCC,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com