Use of DLP in Pre-mRNA splicing and cell cycle control
A cell cycle and sequence technology applied in the field of DLP proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Extraction of total mRNA
[0127] Extraction of total cellular mRNA using Trizol from Invitrogen TM reagent. Take 1×10 each 7 MCF-7 cells, HepG2 and Ishikawa cells were collected by centrifugation, and 1ml Trizol was added TM Reagent, blow repeatedly to break the cells, add 0.2ml chloroform after standing still at room temperature for 5 minutes, shake vigorously for 15 seconds, 12000rpm at 4°C for 15 minutes, collect supernatant and precipitate with isopropanol, wash with 70% ethanol once, and dry at room temperature, Total RNA was resuspended in DEPC-treated H 2 In O, it was used for cDNA first-strand synthesis after quantification by spectrophotometer.
[0128] cDNA duplex synthesis
[0129] Using NEB's ProtoScript TM First Strand cDNA Synthesis Kit uses the mRNA mentioned in 2.3 as a template to synthesize the first and second strands of cDNA. The brief description is as follows: 1ng-2ugmRNA, 2ul Primer dT23VN, 4ul dNTP, add H 2 O to 16ul reaction system, 70°C...
Embodiment 2
[0158] Preparation of anti-GST-DLP polyclonal antibody and Western blot hybridization
[0159]The purified GST-DLP fusion protein was used to immunize New Zealand white rabbits to prepare polyclonal antibodies against DLP. For Western blot, the extracted protein was quantified using Bio-Rad's DC protein quantification reagent, and then subjected to 15% SDS-PAGE electrophoresis. After electrophoresis, the protein was transferred to a nitrocellulose membrane. After the membrane was blocked with 5% skimmed milk powder for 2 hours, a 1:30 dilution of rabbit anti-DLP polyclonal antibody or a 1:5000 dilution of anti-c-myc monoclonal antibody (Invitrogen) was added at 4°C overnight, and washed three times with TBST After) (10 minutes / time), add horseradish enzyme-labeled anti-rabbit IgG (Amersham Pharmacia Biotech) or goat anti-mouse IgG (Santa Cruz) diluted 1:4000, incubate for 1 hour, and wash the membrane three times with TBS Add substrate solution for color development.
Embodiment 3
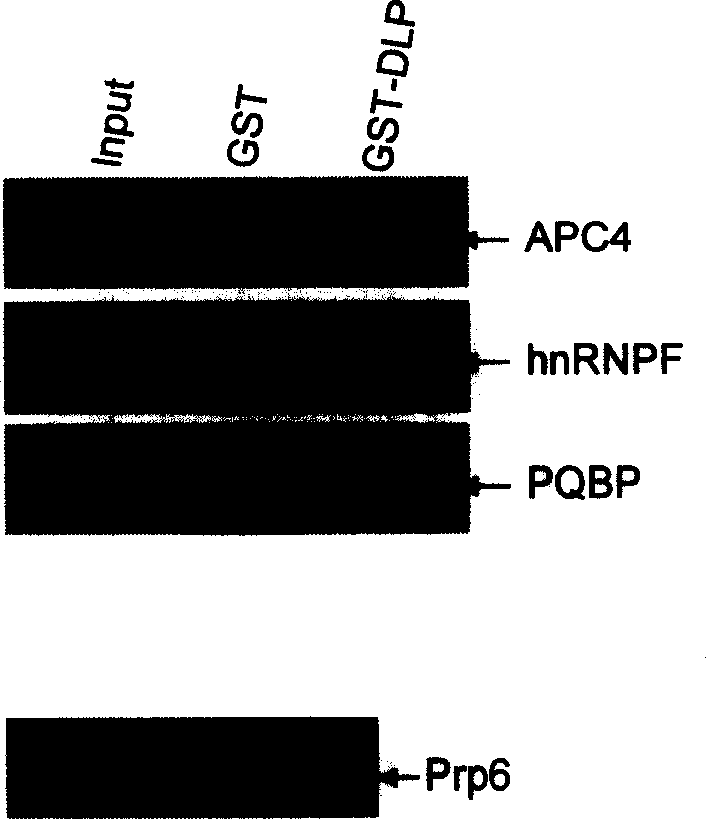
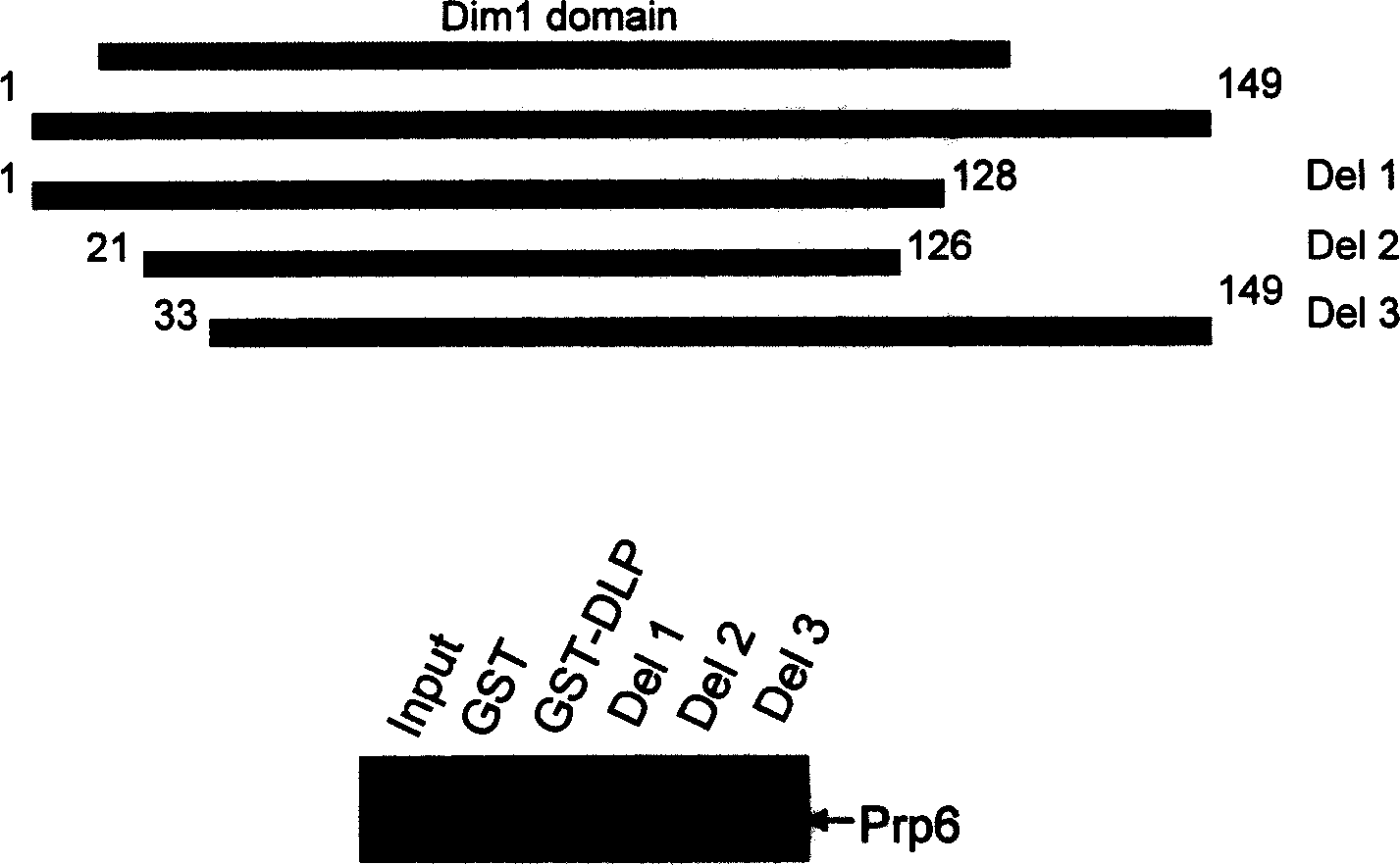
[0161] GST Pull-down
[0162] Using the TNT Rabbit Reticulocyte lysate and TNT T7polymerase labels kit from Promega, pcDNA-Prp6, hnRNPF, PQBP and APC4 were transcribed and translated in vitro. Proceed as follows:
[0163] (1) Add reagents to the 1.5ml tube in the following order
[0164] 25ul TNT Lysate
[0165] 2ul TNT reaction buffer
[0166] 1ul TNT RNA polymerase
[0167] 1ul Amino Acid Mix, minus Met(1mM)
[0168] 2ul [ 35 S] Methionine (>1000ci / mmol)
[0169] 1ul Rnasin Ribonuclease inhibitor
[0170] 2ul pcDNA-Prp6, hnRNPF, PQBP and APC4
[0171] 16ul nuclease-free water
[0172] 50ul total volume
[0173] 30°C for 90 minutes.
[0174] (2) Add 50ul glutathione agarose beads, 500ng-10ug GST or GST-DLP, and 4-5ul in vitro transcription and translation products into a 1.5ml tube.
[0175] (3) Incubate at 4°C for 2 hours, 13000rpm for 2 minutes, discard the supernatant. Wash glutathione agarose beads with 1ml of cold lysis buffer.
[0176] (4) Centrifuge at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com