Yimaikang dispersion tablet and its preparing method
A technology for dispersible tablets and active ingredients, applied in the field of Yimaikang dispersible tablets and their preparation, can solve the problems of slow dissolution, long disintegration time of ordinary tablets, slow onset of effect, etc., and achieves fast onset of effect, good taste and convenient taking. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
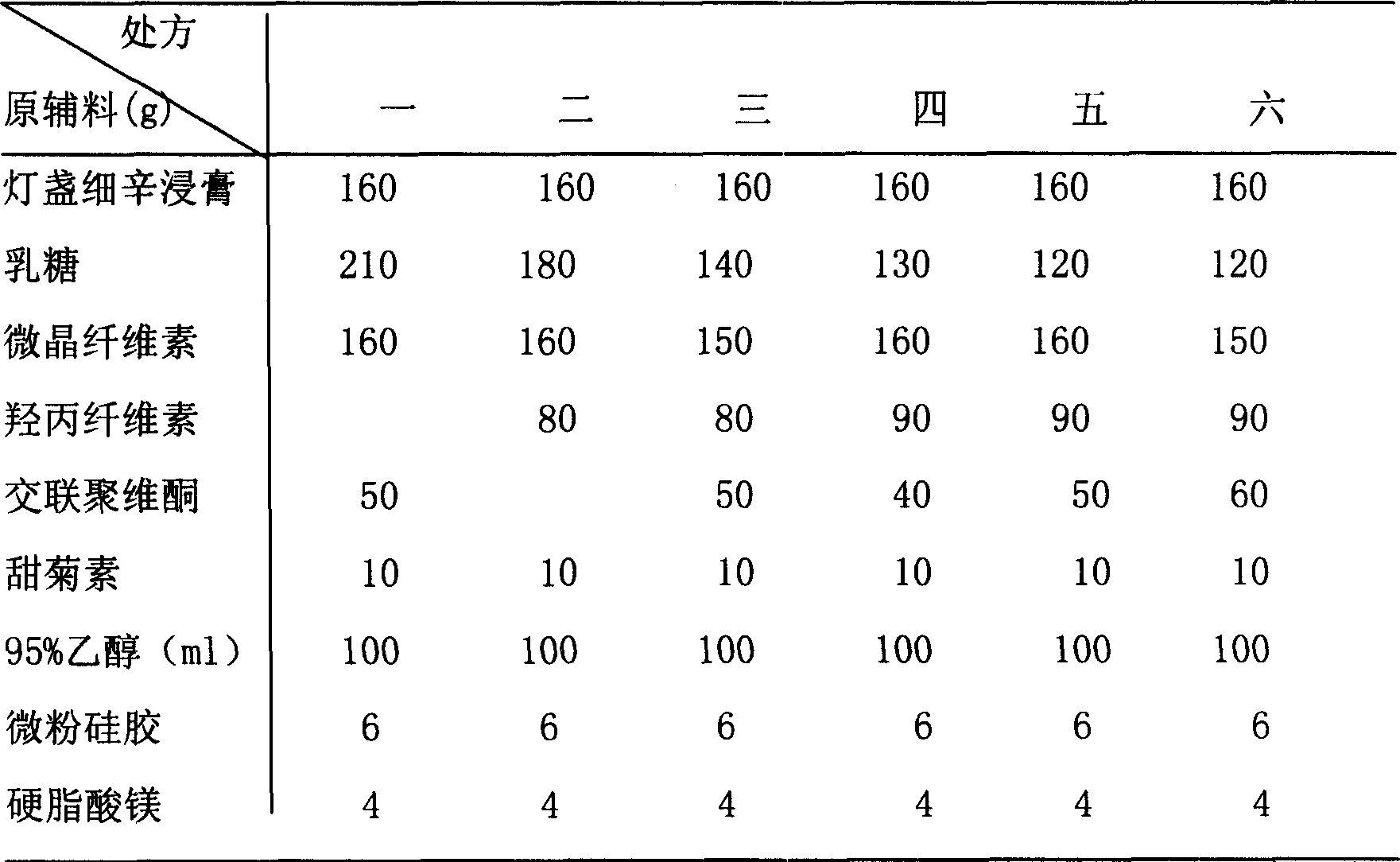
[0050] Erigeron extract 160g
[0051] Lactose 120g
[0052] Microcrystalline Cellulose 150g
[0053] Hypromellose (low substitution) 90g
[0054] Crospovidone 60g
[0055] Stevia 10g
[0056] 95% ethanol 100ml
[0057] Micronized silica gel 6g
[0059] Makes 1000 pieces
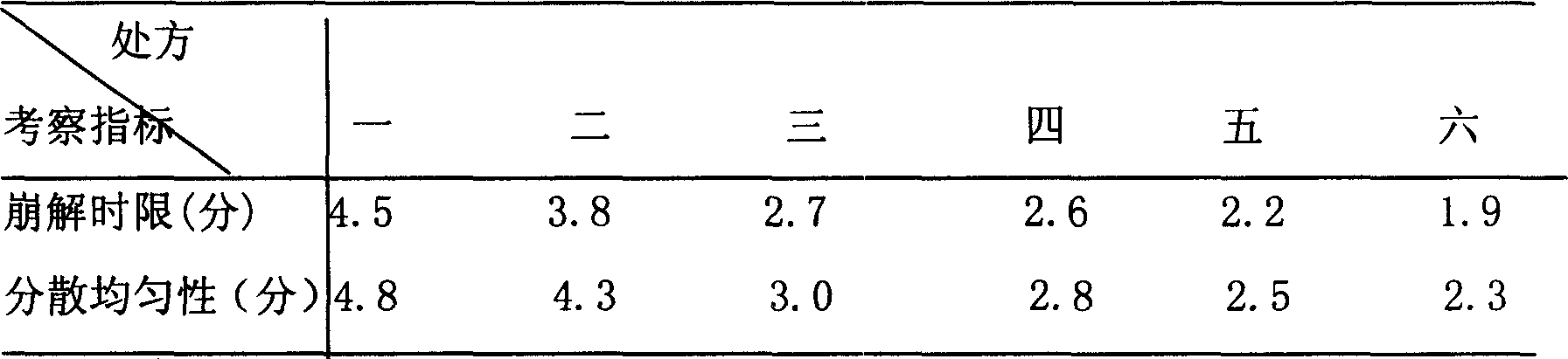
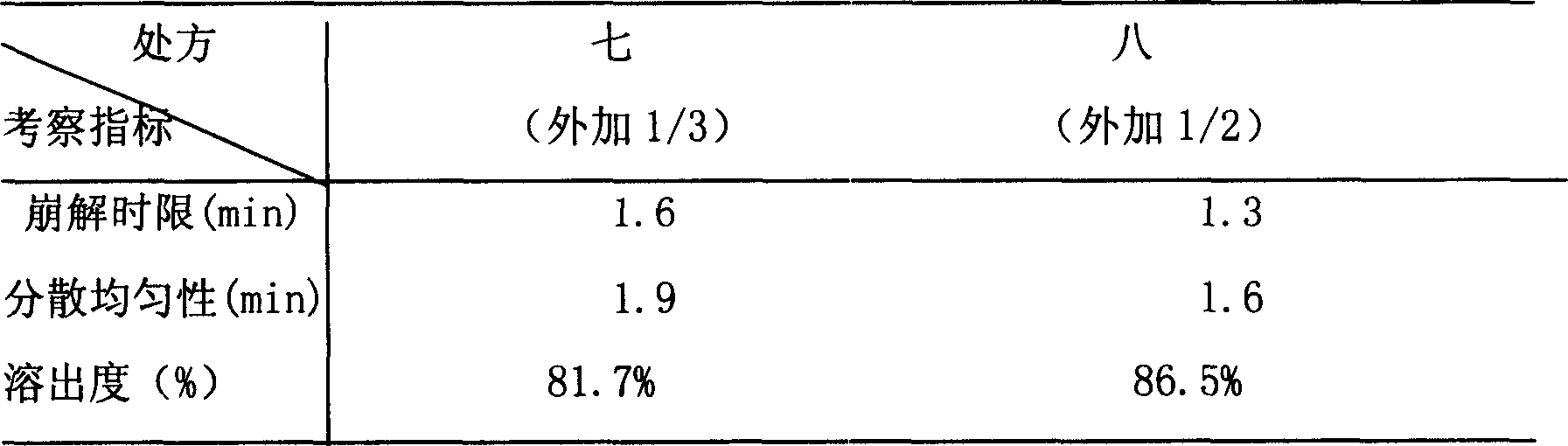
[0060] The raw and auxiliary materials were crushed through a 100-mesh sieve, and the purchased raw materials Erigeron breviscapus extract, lactose, microcrystalline cellulose, hydroxypropyl cellulose (low substitution), crospovidone (1 / 2 amount) were weighed according to the prescription amount ), stevioside, mixed evenly, adding 95% ethanol to make soft material, soft material is granulated with 18 mesh sieve, wet granule is dried at 50 ℃, after drying, granulate with 18 mesh sieve, add crospovidone (1 / 2 quantity), micropowder silica gel, magnesium stearate, mix evenly, measure the content of the semi-finished product, press the...
Embodiment 2
[0062] Erigeron extract 80g
[0063] Lactose 50g
[0064] Microcrystalline Cellulose 50g
[0065] Hypromellose (low substitution) 20g
[0066] Crospovidone 20g
[0067] Stevia 2g
[0068] 95% ethanol 40ml
[0069] Micronized silica gel 2g
[0071] Makes 1000 pieces
[0072] The raw and auxiliary materials were crushed through a 100-mesh sieve, and the purchased raw materials Erigeron breviscapus extract, lactose, microcrystalline cellulose, hydroxypropyl cellulose (low substitution), crospovidone (1 / 2 amount) were weighed according to the prescription amount ), stevioside, mixed evenly, adding 95% ethanol to make soft material, soft material is granulated with 18 mesh sieve, wet granule is dried at 50 ℃, after drying, granulate with 18 mesh sieve, add crospovidone (1 / 2 quantity), micropowder silica gel, magnesium stearate, mix evenly, measure the content of the semi-finished product, press the oval...
Embodiment 3
[0074] Erigeron extract 160g
[0075] Lactose 200g
[0076] Microcrystalline Cellulose 250g
[0077] Hypromellose (low substitution) 150g
[0078] Crospovidone 150g
[0079] Stevia 20g
[0080] 95% ethanol 200ml
[0081] Micronized silica gel 20g
[0083] Makes 1000 pieces
[0084] The raw and auxiliary materials were crushed through a 100-mesh sieve, and the purchased raw materials Erigeron breviscapus extract, lactose, microcrystalline cellulose, hydroxypropyl cellulose (low substitution), crospovidone (1 / 2 amount) were weighed according to the prescription amount ), stevioside, mixed evenly, adding 95% ethanol to make soft material, soft material is granulated with 18 mesh sieve, wet granule is dried at 50 ℃, after drying, granulate with 18 mesh sieve, add crospovidone (1 / 2 quantity), micropowder silica gel, magnesium stearate, mix evenly, measure the content of the semi-finished product, press...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com