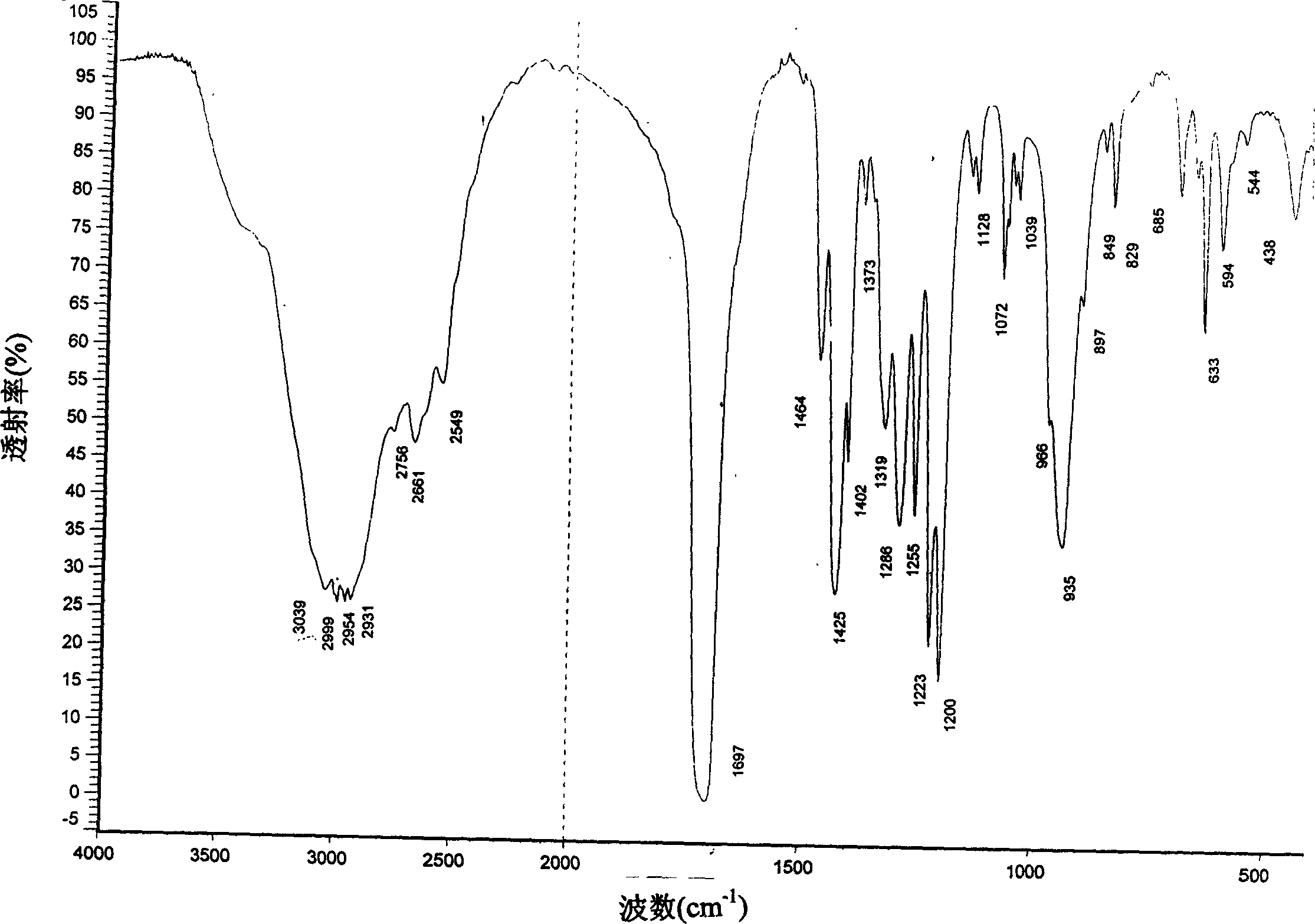
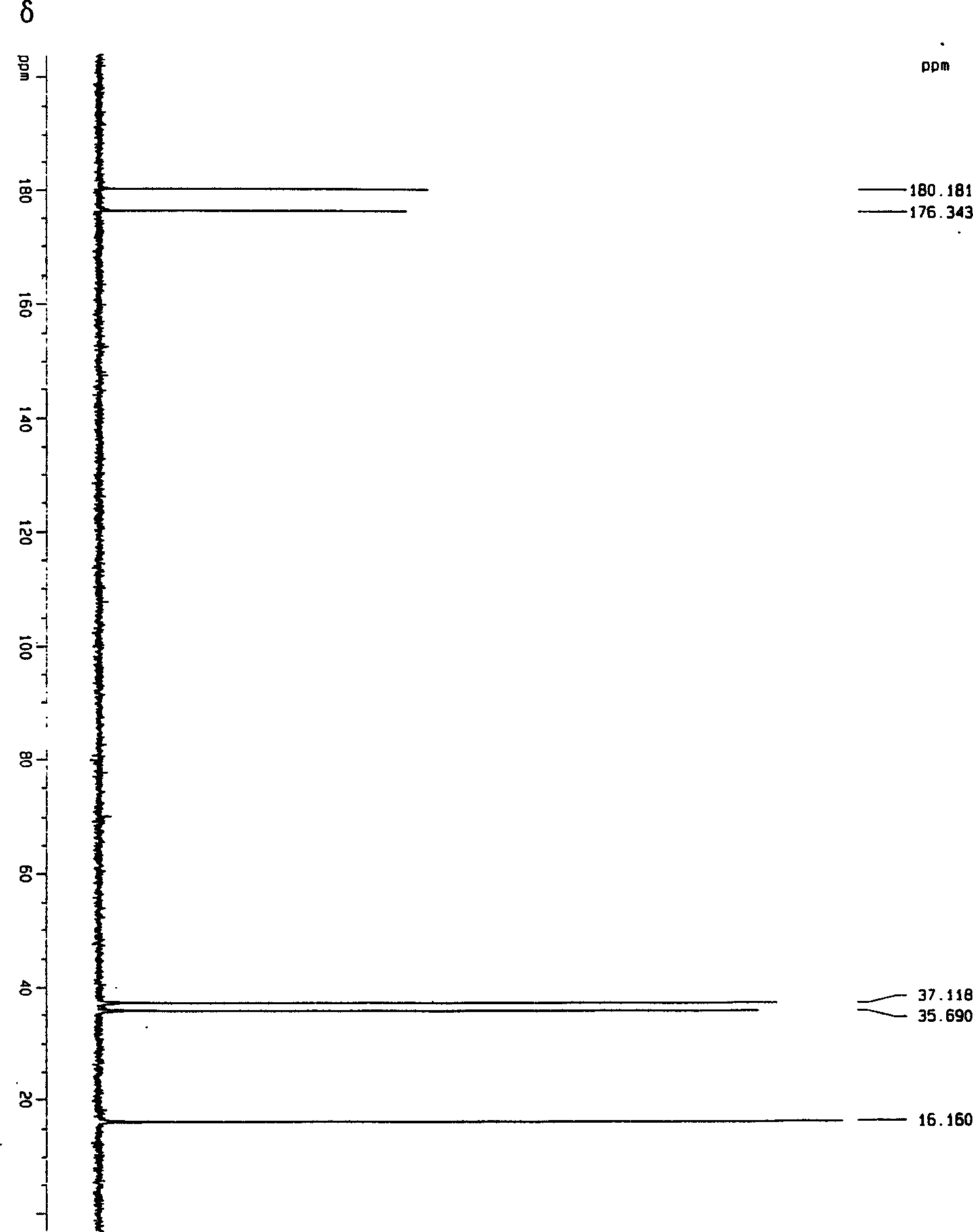
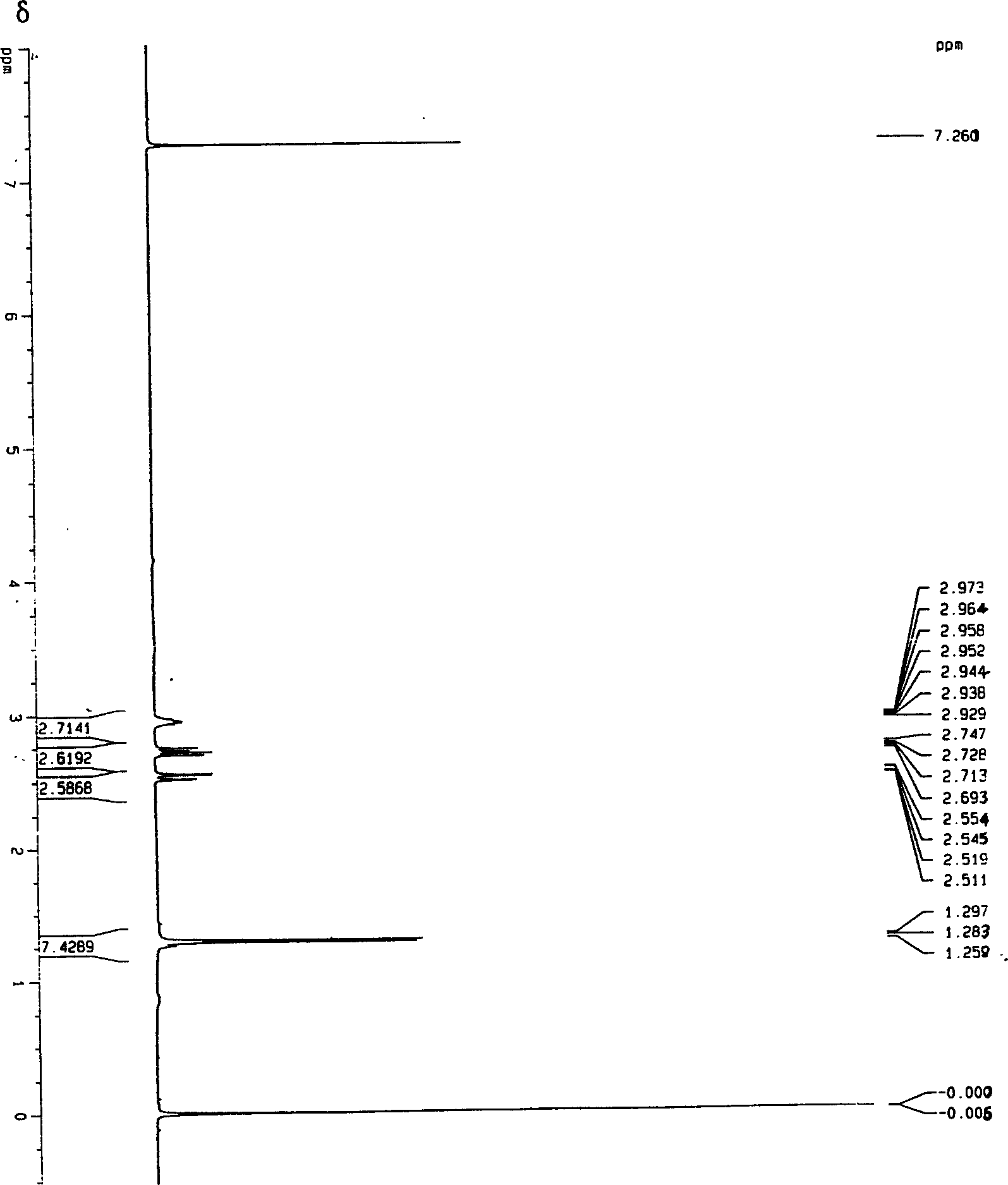
Catalytic synthesis process of methyl succinic acid
A technology of methyl succinic acid and a synthesis method, applied in the field of synthesis of methyl succinic acid, can solve the problems of many operation steps, complicated process and high cost, and achieve the effects of strong catalytic stability, simple operation and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0035] Embodiment: 1 is carried out in long 13 centimeters, the intermittent tank reactor of diameter 3.8 centimetres, puts into palladium carbon catalyst (palladium content 5wt%) 1.0g and 10g raw material itaconic acid, adds mixed solvent 10ml ethanol and 50ml THF, After dissolving, check for leaks, pass hydrogen gas, stir, keep constant temperature at 50°C and constant pressure at 2.5atm for hydrogenation reaction. The reaction was basically completed after 12 hours. Sedimentation, filtration, vacuum distillation, drying. The obtained product has a conversion rate of 99.15% and a selectivity of 99.95%.
[0036] Embodiment: 2 same reaction apparatus, with 18g palladium-carbon catalyst (w0.25%) 10g raw material, mixed solvent ratio is 0ml ethanol / 60ml tetrahydrofuran, 10 ℃, 1.0atm, reacted 16 hours, obtained product transformation rate 99.83%, The selectivity is 99.67%.
[0037] Embodiment: 3 same as above reaction apparatus, with 0.15g (w10%) catalyst 10g raw material, mix...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com