Process for preparing L-ofloxacin and ofloxacin
A technology of levofloxacin and synthesis method, which is applied in the chemical field, can solve problems such as easy occurrence of side reactions, impact on product quality and appearance, long hydrolysis reaction time, etc., and achieve convenient and centralized solvent recovery and application, product quality and appearance Improves, hydrolyzes quickly and completely
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
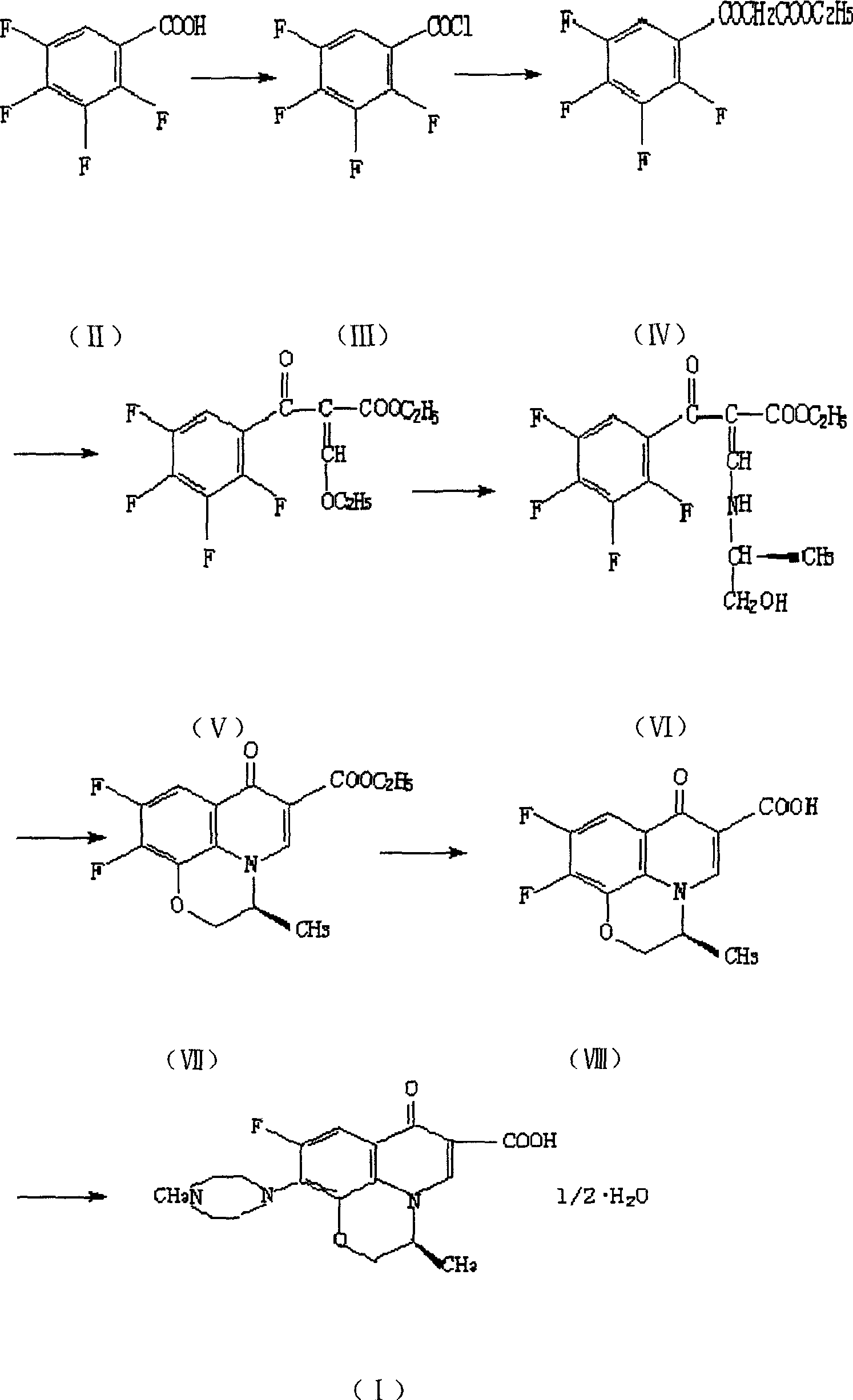
[0030] a) Preparation of compound (IX): Put 60.0g of compound (V) and 150ml of DMF into a 500ml three-necked flask, freeze to 0°C, add 14.0g of L-aminopropanol dropwise, and keep warm for 0.5 hours, then add 45.0 gK2CO3, react at 60-70°C for 3 hours, add 30.0g N-methylpiperazine to the mother liquor, stir and react at 70-80°C for 2 hours, and recover N-methylpiperazine under reduced pressure. Raise the reaction temperature to 120-130°C. After 0.5 hours, the reaction was completed. The reaction solution was poured into a 1000ml beaker equipped with 500ml water, stirred, and a white solid was precipitated. After cooling, filtering, and drying, 43.0g of compound (IX) was obtained. Yield 57.9%.
[0031] b) preparation of levofloxacin (I): 50.5g compound (IX), 150ml water, 50ml concentrated hydrochloric acid obtained in dropping into a step in a 500ml three-necked bottle successively, stir and reflux for half an hour, and the solid dissolves completely, The reaction is over. Afte...
Embodiment 2
[0033] The difference from Example 1 is that isoamyl alcohol is used as the solvent, and the alkali added is sodium bicarbonate. The yield obtained in step a is 45.7%, and the yield obtained in step b is 92.3%.
Embodiment 3
[0035] a) Preparation of compound (X): Put 60.0g of compound (V) and 150ml of DMF into a 500ml three-necked flask successively, freeze to 0°C, add 14.0g of DL-aminopropanol dropwise, and keep warm for 0.5 hours, then add 45.0 gK2CO3, react at 60-70°C for 3 hours, add 30.0g N-methylpiperazine to the mother liquor, stir and react at 70-80°C for 2 hours, and recover N-methylpiperazine under reduced pressure. Raise the reaction temperature to 120-130°C. After 0.5 hours, the reaction is over. The reaction solution is poured into a 1000ml beaker filled with 500ml of water, stirred, and a white solid is precipitated. After cooling, filtering, and drying, 45.0g is obtained, with a yield of 60.6%. .
[0036] b) Preparation of Ofloxacin (XI): 52.0 g of solid compound, 150 ml of water, and 50 ml of concentrated hydrochloric acid obtained in step a were dropped into a 500 ml three-necked bottle successively, stirred and refluxed for half an hour, and the reaction was completed. After the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com