Alkannin derivatives as immune inhibitors and metal complexes thereof
A technology of metal complexes and immunosuppressants, applied in calcium organic compounds, zinc organic compounds, copper organic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
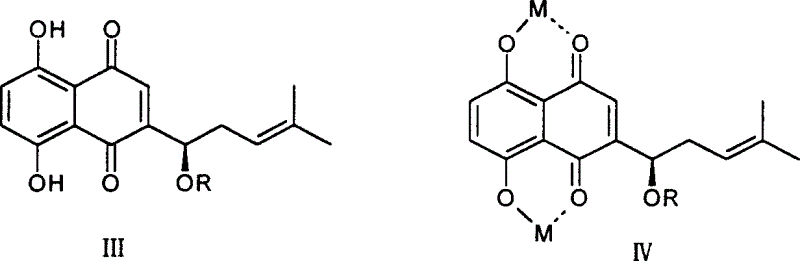
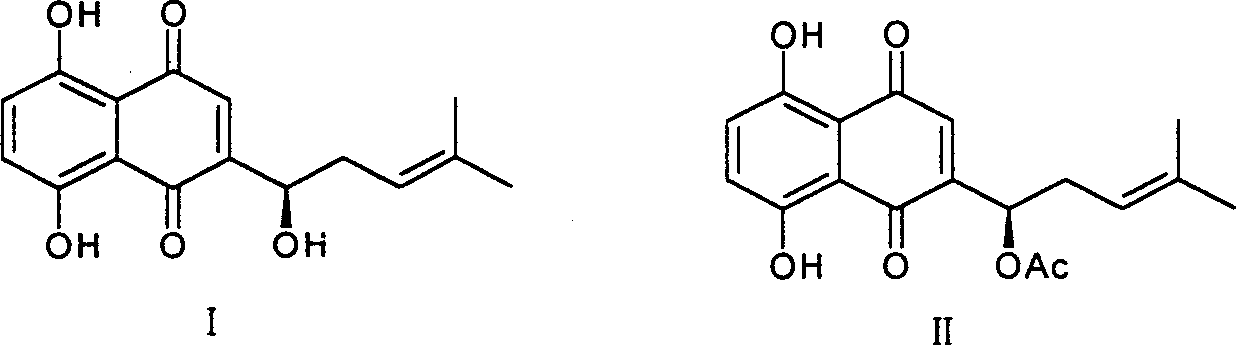
[0018] 1. Preparation of shikonin
[0019] CO 2 10 grams of supercritical extract, dissolved in 500ml of 0.2mol / l NaOH solution, stirred at room temperature for 24 hours, centrifuged to remove insoluble matter, combined supernatant, added 0.5mol / l H 2 SO 4 Solution until the solution turns red, put it aside for 2 hours, centrifuge to obtain a red precipitate, wash with water, and dry to obtain 5 g of shikonin crude product, recrystallize from petroleum ether to obtain 2.5 g of reddish-brown metallic luster crystals, yield 25% . Melting point 148-149°C, H-NMR (CDCl 3 )δ: 1.651, 1.755 (s, each 3H, 2CH 3 ), 2.311-2.387 (1H, m, -CHa), 2.623-2.660 (1H, m, -CHb), 4.911 (1H, d, J = 7.2, 4.0, -OCH-), 5.202 (1H, dd, J =8.0, 6.8, =CH-), 7.165 (s, 1H, Quin-H), 7.192 (2H, 2Ar-H), 12.494, 12.598 (each 1H, s, 2Ar-OH), consistent with literature reports (plant Acta Sinica, 1989, 31(7), 549-553).
[0020] 2. Preparation of shikonin ester derivatives
[0021] General method: take sever...
example
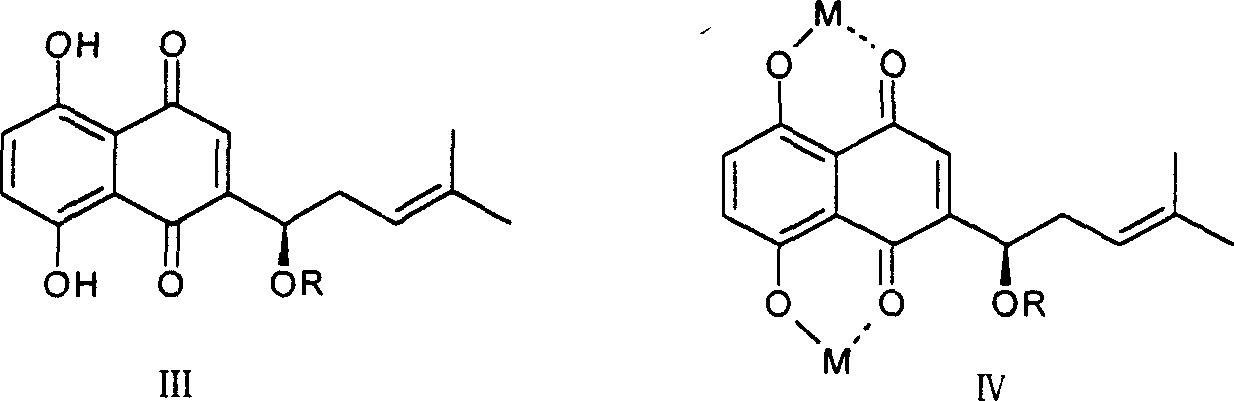
[0028] (1) Acetylshikonin copper complex: take 176mg of acetylshikonin, dissolve it in 3ml of acetone, and take another 194mg of copper acetate, dissolve it in 5ml of water, mix the two, place, precipitate out, filter, wash with a small amount of water, After drying, 229 mg of the complex was obtained, with a yield of 94.2%.
[0029] (2) Acetylshikonin zinc complex: Take 236mg of acetylshikonin, dissolve it in 5ml of acetone, and take another 263mg of zinc acetate, dissolve it in 5ml of water, mix the two, let it stand, precipitate out, filter, wash with a small amount of water, After drying, 309 mg of the complex was obtained, with a yield of 93.8%.
[0030] (3) Acetylshikonin calcium complex: Take 250mg of acetylshikonin, dissolve it in 5ml of acetone, and take another 240mg of calcium acetate, dissolve it in 8ml of water, mix the two, let it stand, precipitate out, filter, wash with a small amount of water, After drying, 298 mg of the complex was obtained, with a yield of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com