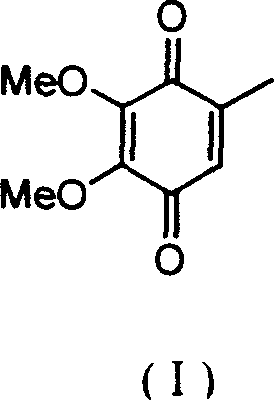
2,3-dimethoxy-5-methyl-1,4-benzoquinone ú¿ó±ú®preparation method
A technology of trimethoxytoluene and dimethoxy, which is applied in 2 fields, can solve the problems of large corrosion of industrial equipment, unsuitable for large-scale industrial production, corrosion of industrial equipment, etc., and achieves the effects of less environmental pollution, easy recycling and application, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
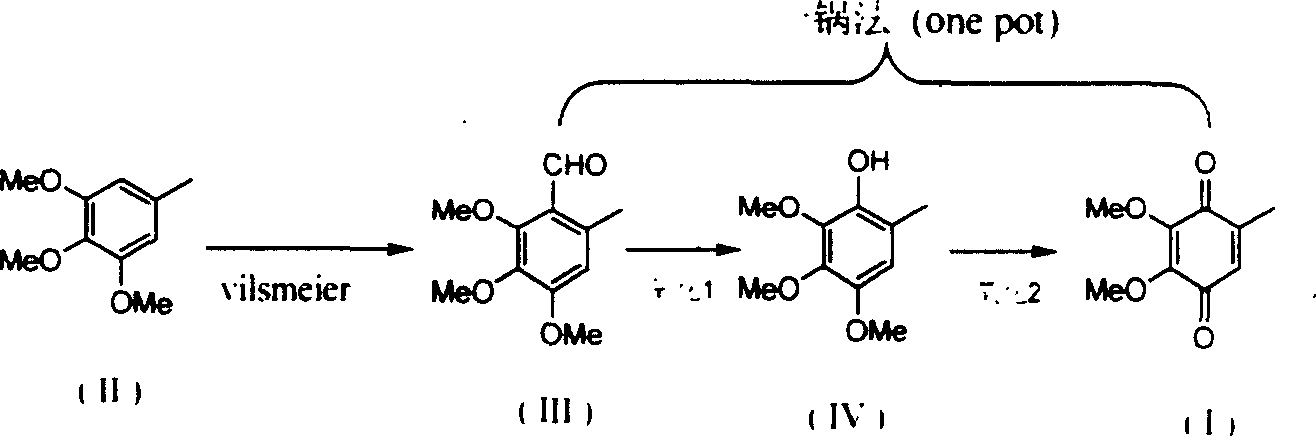
[0014] (1) Preparation of 2,3,4-trimethoxy-6-methyl-benzaldehyde
[0015] Embodiment (1): 250ml dry three-necked flask is placed in ice-water bath, adds 120 grams (0.66mol) of 3,4,5-trimethoxytoluene and 76ml (72g, 0.99mol) of dimethylformamide, mechanical stirring Let it dissolve completely. Slowly add 72 ml of phosphorus oxychloride (120 g, 0.78 mol) dropwise thereto, stir at 30°C for 2 h after the addition, and then raise the temperature to 90°C for 1 h to complete the reaction to obtain a brown-red viscous liquid. Slowly pour it into ice water, add 2N aqueous sodium hydroxide solution under stirring until the solution is weakly alkaline, collect the precipitated white solid, wash with water, and dry in vacuo to obtain white solid 2,3,4-trimethoxy-6-methanol Base-benzaldehyde 133g, yield 96%, Mp: 61°C.
[0016] Example (2): A 100ml dry three-necked flask was placed in an ice-water bath, 12g (0.065mol) of 3,4,5-trimethoxytoluene and 15ml of dimethylformamide were added, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com