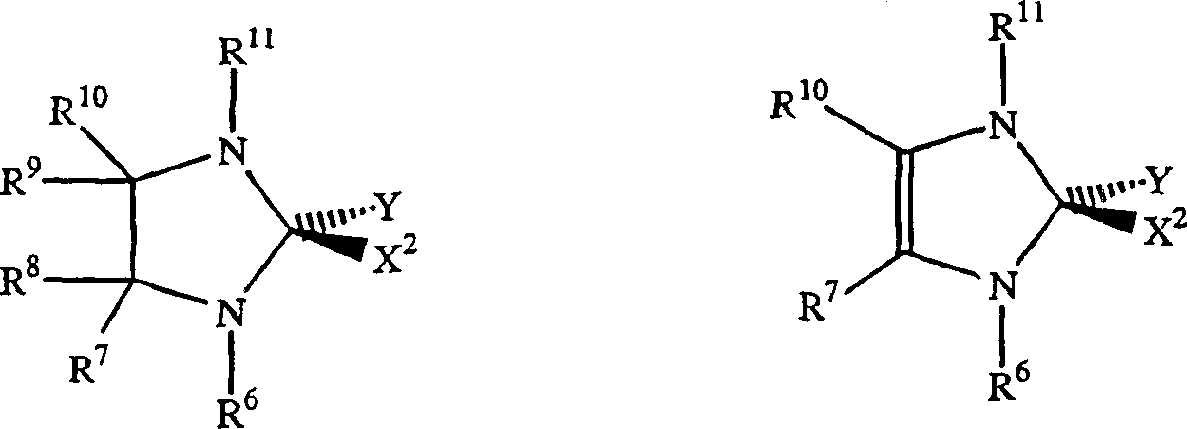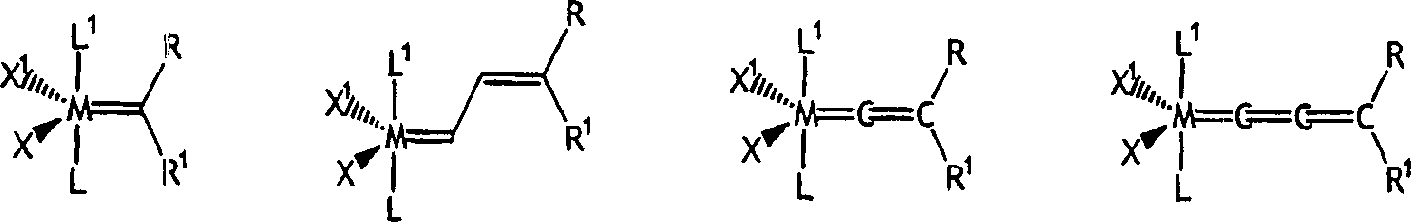High activity metal carbene metathesis catalysts generated using thermally activated N-heterocyclic carbene precursor
A heterocyclic carbene and catalyst technology, applied in the field of highly active metal carbene metathesis catalysts prepared by thermally activating N-heterocyclic carbene precursors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0160] Can be used with NHC-X 2 -The preferred examples of Y mixed Ru or Os initiators are as follows:
[0161]
[0162] Where M = Ru or Os.
[0163] X 1 And X 2 Represents any anionic ligand, independently selected from chlorine (Cl), bromine (Br), iodine (I), thiocyanate (SCN), cyanate (CN), carboxylate (OC(O)R), trifluoro Acetate (OC(O)CF 3 ), trifluoromethanesulfonate (O 3 SCF 3 ), triflimide(N(SO 2 CF 3 ) 2 ), acetylacetone (acac), alkoxide (RO), aryl ether (ArO) and tosylate (O 3 SC 6 H 4 CH 3 ).
[0164] X is a functional group that can bond with the metal center, and the functional group is also bound to the alkylene part of the initiator (the "initiation point" and the subsequent "catalyzed" ring opening reaction of the strained ring) through the carbon skeleton; and wherein X is selected From alkoxy (-OR), thiooxy (-SR), phosphine (-PR) 2 ), phosphine (-P(O)R 2 ), amido (-NR 2 ), arsenic (-AsR 2 ), (-Sb 2 ), ene (-CR=CR 2 ), alkyne (-CCR), carboxylate (-OC(O)R), aceta...
Embodiment 1
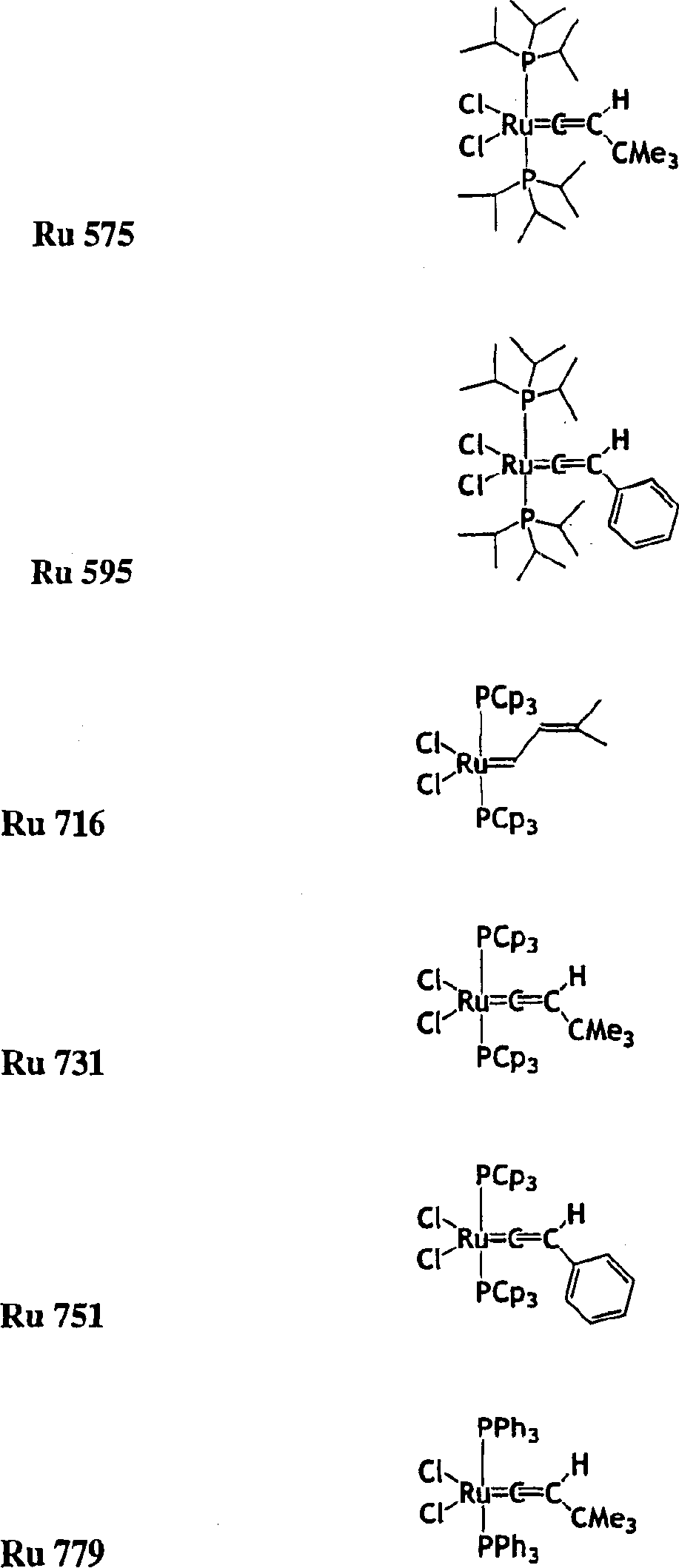
[0300] The mixture was heated to the starting temperature of 49 ℃, in s-ImesHCCl 3 In the presence of =0.0215g, use Ru716 =0.0361g to polymerize 50g of DCPD (containing 8% by weight of trimeric DCPD), DCPD:Ru:s-ImesHCCl 3 The ratio of reagents is 7500:1:1.
[0301] Result: the time required to reach the maximum temperature (T max )=81s. T max =227°C. Conversion rate measured by thermogravimetric analysis (TGA) = 97.35%. Glass transition temperature measured by thermal mechanical analysis (TMA) = 154°C. Residual monomer ratio (extracted with toluene at room temperature)=0.51%.
Embodiment 2
[0303] The mixture was heated to the starting temperature of 49 ℃, in s-ImesHCCl 3 In the presence of =0.0041g, use Ru716 =0.00677g to polymerize 50g of DCPD, DCPD:Ru:s-ImesHCCl 3 The ratio of reagents is 40,000:1:1.
[0304] Result: the time required to reach the maximum temperature (T max )=510s. T max =192°C. The conversion rate measured by thermogravimetric analysis (TGA) at 400°C under nitrogen = 87.53%. Glass transition temperature measured by thermal analysis method (TMA) = 105°C. Residual monomer ratio (extracted with toluene at room temperature) = 9.74%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com