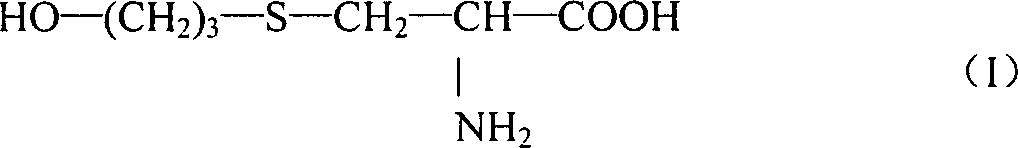Liquid drug preparations
A technology of liquid preparations and drugs, which is applied in the direction of drug combination, drug delivery, and pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Studies on the effect of pH on liquid formulations of fudosteine:
[0024] Adjust the pH of the aqueous solution containing 8 g of fudosteine with an appropriate amount of phosphoric acid or sodium hydroxide, and adjust the pH to 2-7 respectively as shown in Table 1. Purified water was added to bring the total amount of each solution to 100 ml, thereby obtaining fudosteine liquid preparations with different pH values. The liquid preparation was stored at 70° C. for 7 days, and the residual rate of fudosteine in the solution and whether there was discoloration and precipitation were detected. The content of fudosteine was quantitatively analyzed by high performance liquid chromatography (HPLC), thereby calculating the residual rate of fudosteine. Visually inspect for discoloration and precipitation, and evaluate the results according to the following criteria. The results are shown in Table 1.
[0025] Discoloration Evaluation Criteria:
[0026] comment conte...
Embodiment 2
[0040] Study on the Types of pH Regulators for Fudosteine Liquid Preparations
[0041] An aqueous solution containing 8 g of fudosteine was adjusted to pH 3.5 by adding various acids as shown in Table 2. Purified water was added to bring the total amount of each solution to 100 ml, whereby a fudosteine liquid preparation was obtained. This liquid preparation was stored at 70°C for 7 days, and the presence or absence of discoloration was evaluated in the same manner as in Example 1. The results are shown in Table 2.
[0042] (result)
[0043] Analysis value
[0044] The results confirmed that both inorganic acids and organic acids can be used in the pharmaceutical liquid formulations of the present invention. In addition, the pH of each solution was hardly changed by the acid after 7 days, that is, the change in pH was within the allowable error range.
Embodiment 3
[0046] Studies on types of sweeteners used in liquid formulations of fudosteine:
[0047] An aqueous solution containing 8 g of fudosteine and 20 g of the sweeteners shown in Table 3 was adjusted to pH 3.5 by adding phosphoric acid. Purified water was added to bring the total amount of each solution to 100 ml, whereby a fudosteine liquid preparation was obtained. This liquid preparation was stored at 70°C for 7 days, and the presence or absence of discoloration was evaluated in the same manner as in Example 1. The results are shown in Table 3.
[0048] (result)
[0049] Analysis value
Survival rate (%)
discoloration
Refined white sugar
90.5
++
Glucose
78.8
++
90.7
++
isomerized sugar
90.8
++
D-sorbose
100.7
-
D-Mannitol
99.3
-
100.3
-
99.5
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com