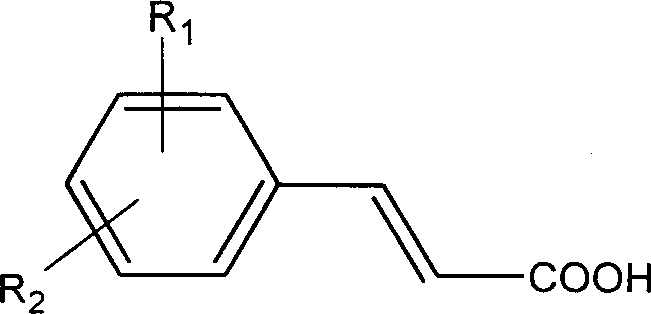
Organosilicon modified cinnamate as reactive ultraviolet ray absorbent and its prepn and application
A technology of cinnamate and cinnamate is applied in the application field of preparing organosiloxane polymers and composite anti-ultraviolet materials, and can solve the problems of poor compatibility, few applications, small molecular weight and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Add 21.6g (0.1mol) of potassium p-methoxycinnamate and 60g of N,N-dimethylformamide into a four-necked bottle equipped with an electric stirrer, a thermometer, a distillation device, and a dropping funnel, and heat and stir to make It dissolves completely. Add 21.8 g (0.11 mol) of γ-chloropropyltrimethoxysilane, raise the temperature to 130° C., change the distillation device to a reflux device, and react for 10 hours. Cool, let stand overnight, and filter with suction. The filtrate was distilled under reduced pressure by a water pump under the protection of nitrogen, and the solvent N,N-dimethylformamide was recovered.
[0043] Cool down, change the oil pump. Distill under reduced pressure under the protection of nitrogen, and collect fractions at 189-191°C / 50Pa. 24.5 g of 3-trimethoxysilylpropyl p-methoxycinnamate was obtained, with a yield of 72.1%. IR (liquid film method): 3060 (γ =C-H , benzene ring); 2944, 2840 (γ C-H -CH 2 -, -CH 3 );1710(γ C=O );1635(γ ...
Embodiment 2
[0047] Add 18.3g (0.1mol) of m-chlorocinnamic acid and 45.0g of N,N-dimethylformamide into a four-neck flask equipped with an electric stirrer, a thermometer, a distillation device, and a dropping funnel, and heat and stir to dissolve it completely . Slowly add 18.0 g (0.1 mol) of 30% industrial sodium methoxide methanol solution, add 21.8 g (0.11 mol) of γ-chloropropyltrimethoxysilane, recover methanol under heating and stirring, and raise the temperature to 110°C. Change the distillation device to a reflux device, control the temperature at 110°C, and react for 20 hours. Let cool and let stand overnight. Suction filtration, under the protection of nitrogen, the filtrate was distilled under reduced pressure by a water pump, and 35 g (N,N-dimethylformamide) of a fraction of 48-50 ° C at 730 mmHg was collected. Under this pressure, the oil bath was heated to 180 ° C, and no Fractions distilled. Cool down, change the oil pump. Distilled under reduced pressure under the prote...
Embodiment 3
[0051] Add 2,3-dimethoxycinnamic acid 10.4g (0.05mol) and N,N-dimethylformamide 20g in the four-neck flask equipped with electric stirrer, thermometer, reflux device, dropping funnel, stir Heated to 50°C, dissolved into a transparent solution, and slowly added 9 g (0.05 mol) of 30% industrial sodium methoxide methanol solution. Distill methanol under heating and stirring, change the distillation device to a reflux device, raise the temperature to 160°C, add 10.9 g (0.055 mol) of γ-chloropropyltrimethoxysilane, and react at this temperature for 10 hours. Let cool, let stand overnight, and divide into two layers. Suction filtration, under the protection of nitrogen, the filtrate was distilled under reduced pressure by a water pump, and 15.0 g (N, N-dimethylformamide) of a fraction of 48-50 ° C at 730 mmHg was collected. Under this pressure, the oil bath was heated to 180 ° C. No There are distillates.
[0052] Cool down, change the oil pump. Distilled under reduced pressure u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com