Preparation method of lithium transition metal oxide
A lithium transition metal and transition metal technology, applied in the field of preparation of lithium transition metal oxides, can solve the problems of cumbersome operation, complicated process, difficult implementation, etc., and achieve the effects of uniform particle size distribution, uniform composition, and simple manufacturing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
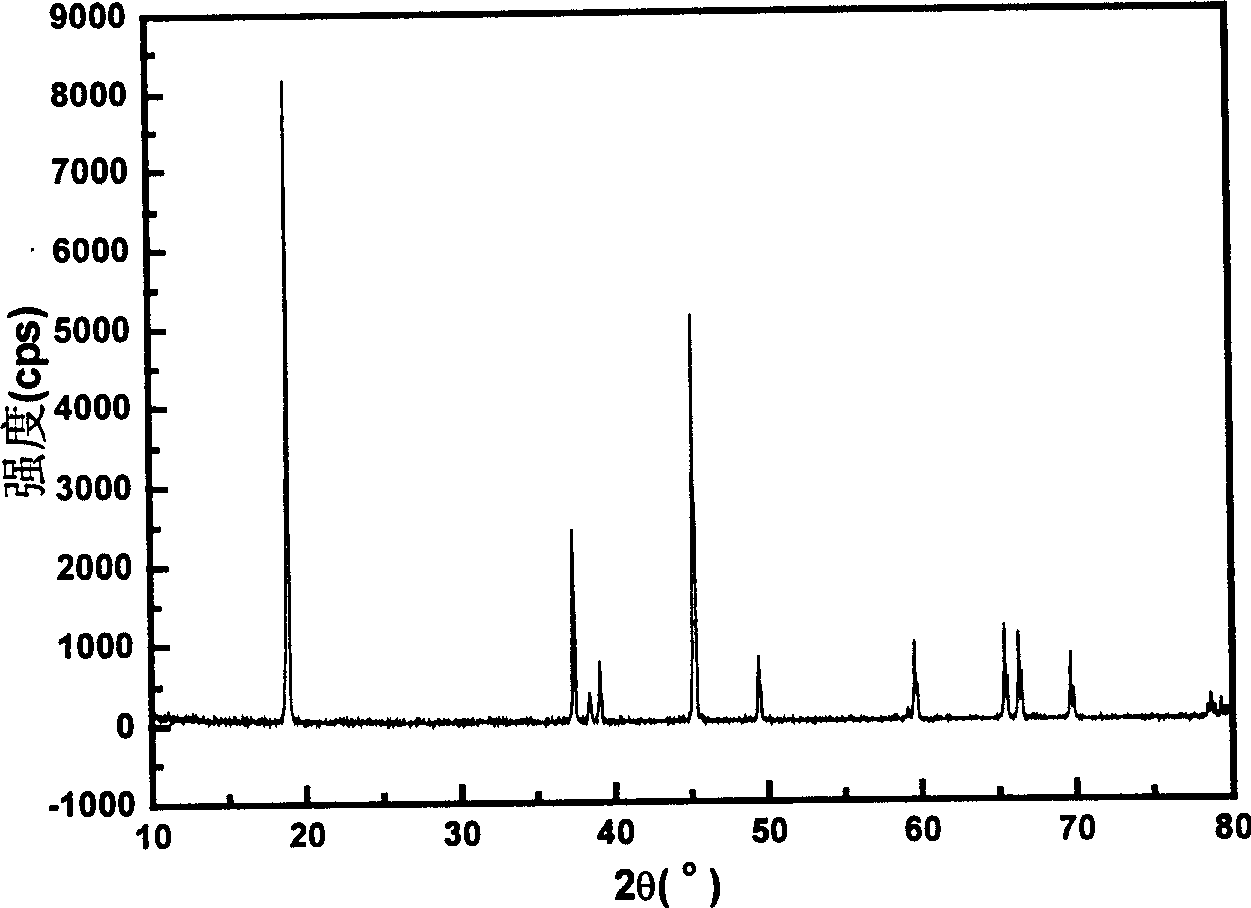
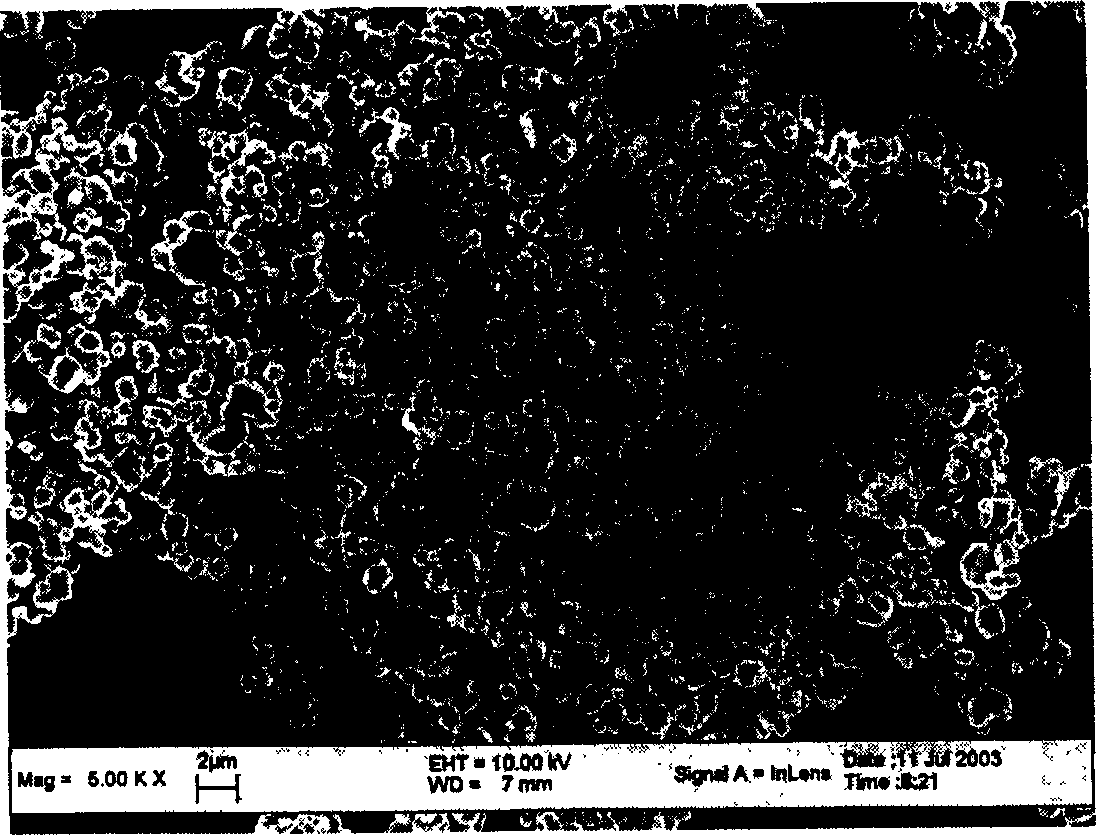
[0025] LiOH·H 2 O, CoO and KNO 3 Mix evenly at a molar ratio of 1:1:3, heat up to 800°C at a rate of 5°C / min in an air atmosphere, roast at a constant temperature for 1 hour, and then cool naturally with the furnace. Wash first with distilled water, then with ethanol, filter and dry at 130°C. The obtained product was shown to be pure phase LiCoO by XRD analysis 2 The particle size of the compound and the material is 1-2 μm. Charge and discharge between 2.7 and 4.3V, the specific current density is 200mA / g, the initial charge specific capacity is 141mAh / g, the discharge specific capacity is 132mAh / g, the efficiency is 93.6%, and the cycle performance is good.
Embodiment 2
[0027] Will Co(NO 3 ) 2 ·6H 2 O, Ni(NO 3 ) 2 ·6H 2 O, MnCl 2 4H 2 O is made into an aqueous solution with a molar ratio of 1:1:1, and is dropped into an excess KOH aqueous solution to obtain a mixture of Co, Ni, and Mn hydroxides (CoNiMn) 1 / 3 (OH) 2 , the precipitate was washed with distilled water until neutral, filtered and dried.
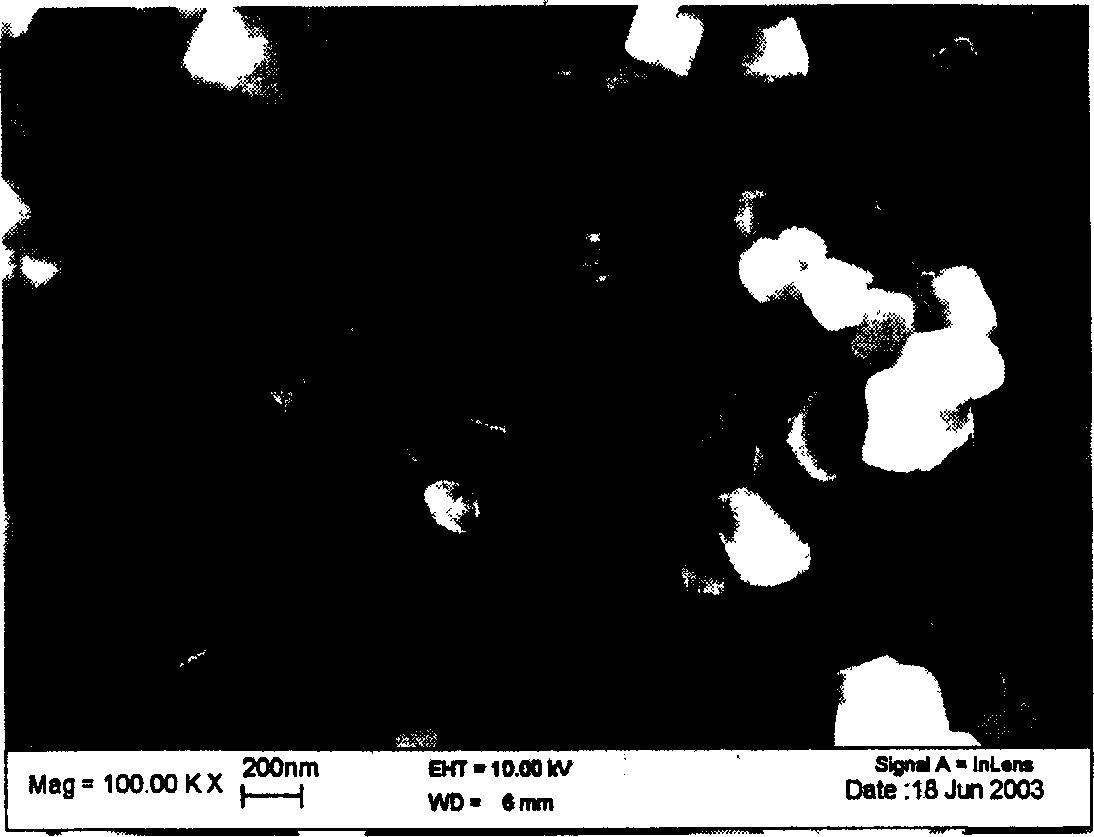
[0028] LiOH·H 2 O, (CoNiMn) 1 / 3 (OH) 2 Mix with KCl at a molar ratio of 1.1:1:4, raise the temperature to 850°C at a rate of 10°C / min in an air atmosphere, roast at a constant temperature for 6 hours, and then cool down to room temperature at a rate of 20°C / min. Wash with distilled water, then with ethanol, filter and dry under vacuum at 100°C. The obtained product was shown to be pure phase Li(CoNiMn) by XRD analysis 1 / 3 o 2 compound, the particle size of the material is about 200nm. Charge and discharge between 2.5 and 4.35V, the specific current density is 1000mA / g, the first discharge can reach 115mAh / g, and the capacity decay ...
Embodiment 3
[0030] LiOH·H 2 O, CoO and KNO3 Mix well at a molar ratio of 1.15:1:2, heat up to 400°C at a rate of 1°C / min in an air atmosphere, roast at a constant temperature for 24 hours, and then cool down to room temperature at a rate of 1°C / min. Wash with distilled water, then with n-butanol, filter and dry at 130°C. The obtained product was shown to be pure phase LiCoO by XRD analysis 2 The particle size of the compound and the material is 50-100nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Graininess | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
| Graininess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com