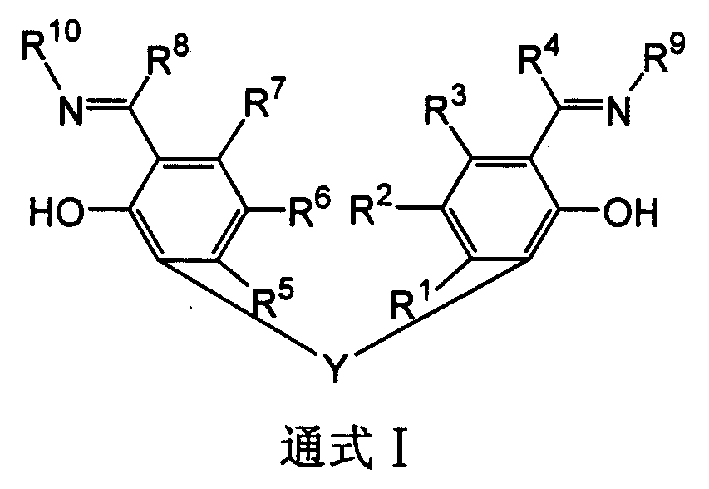
Ligand of non-cyclopentadienyl single active center catalyzer for olefinic polymerization and transition metal complex
A technology of olefin polymerization and transition metals, which is applied in the application field of olefin polymerization catalysts in olefin polymerization, and can solve problems such as restricting the application field of polymers, affecting the processing performance of polymers, and narrow molecular weight distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Synthesis of Ligand L1
[0056] (1) Synthesis of 4,4'-dimethyl-2,2'-propylene-bisphenol
[0057] Add 128ml (600mmol) of p-cresol and 8.8ml (60mmol) of n-propionaldehyde in a 250ml three-necked flask, stir, and add 8ml of concentrated hydrochloric acid dropwise when heated to 63°C, the solution immediately turns yellow, and the temperature rises to 85°C , maintain the reaction for 5 hours, it is an orange-red solution, distill it under reduced pressure, dissolve the residue with a mixed solvent of 80ml chloroform: 80ml petroleum ether, after the precipitate is precipitated, filter the precipitate, and dry it in vacuum to obtain milky white 4,4'-di Methyl-2,2'-propylene-bisphenol product.
[0058] (2) Synthesis of 5,5'-dimethyl-3,3'-propylene-disalicylaldehyde
[0059] Under the protection of nitrogen, add 7.68g (30mmol) of 4,4'-dimethyl-2,2'-propylene-bisphenol synthesized above into a 250ml three-necked flask, dissolve with 60ml of refluxed benzene, stir, At room tem...
Embodiment 2
[0076] Synthetic Ligand L2
[0077] Under a nitrogen atmosphere, add 0.51g (1.63mmol) of 5,5'-dimethyl-3,3'-propylene-disalicylaldehyde synthesized according to the method in Example 1 into a 250ml three-necked flask, and dissolve it in 40ml of methanol , and then add 0.45ml (3.91mmol) of cyclohexylamine and 0.2ml of formic acid, and stir at room temperature for 24 hours. The precipitate was filtered off and dried in vacuo to obtain 0.48 g (1.04 mmol, 75.4% yield) of Ligand L2 as a yellow powder.
[0078] Its structural formula is as follows:
[0079] Ligand L2
[0080] EI-mass spectrum: 474 M +
Embodiment 3
[0082] Synthesis of metal complexes (L1) 3 Zr 2 Cl 4
[0083] Under a nitrogen atmosphere, add 0.70g (1.52mmol) of the ligand L1 synthesized according to the method in Example 1 to a three-neck flask, add 30ml of tetrahydrofuran to dissolve, then cool down to below -70°C, and slowly add 1.33ml (2.13mmol) of n-butyl Lithium-based solution, react at this temperature for 1 hour, slowly warm up to room temperature, and react for 4 hours; transfer this solution to a constant pressure dropper, and slowly add it dropwise until 0.24g (1.00mmol) of the solution is dissolved below -70°C ZrCl 4 In the 30ml tetrahydrofuran solution, after dripping, gradually rise to room temperature, then react for about 18 hours, then, reflux reaction for 5 hours; vacuum distillation, after evaporating to dryness, dissolve with 40ml dichloromethane, filter out insoluble matter, The filtrate was evaporated to dryness under reduced pressure, washed with anhydrous ether, and sucked dry to obtain 0.70 g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com