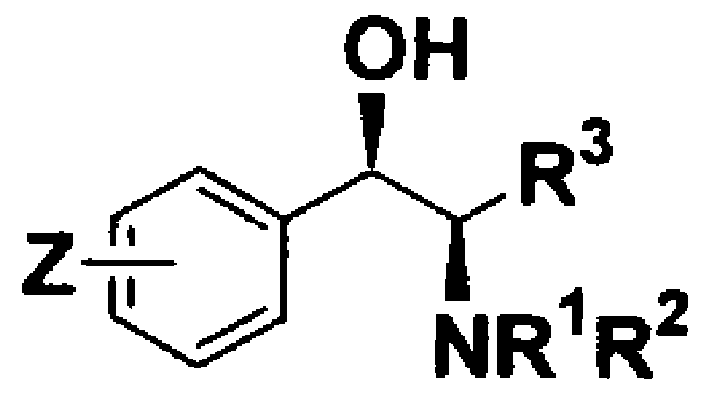
Method of Chiral alkamine ligand used as catalyst of asymmetric addition process for terminal alkyne to fluoroalkylaryl ketone
A chiral amino alcohol, asymmetric technology, applied in the preparation of amino hydroxy compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of difficult industrialization and harsh conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1R, 2R)-2-N, the preparation of N-dimethylamino-3-p-nitrophenyl-1,3-propanediol:
[0033] Reference Jiang, B.; Chen, Z.L.; Tang, X.X.Org.Lett.2002, 4, 3451. Synthesis
Embodiment 2
[0035] (1R, 2R)-3-tert-butoxy-2-N, the preparation of N-dimethylamino-1-p-nitrophenyl-1-propanol:
[0036] At 0-5°C, add 0.8 g of concentrated sulfuric acid dropwise into (1R, 2R)-2-N, N-dimethylamino-3-p-nitrophenyl-1,3-propanediol (1.8 g, 7.5 mmol) in CH 2 Cl 2(20mL) solution. Isobutene gas was maintained at 0-5°C for one hour. Then 0.2 g of concentrated sulfuric acid was added dropwise, the mixture was returned to room temperature and stirred vigorously for 5-7 h, and isobutene gas was continuously introduced. Cool the mixture to 0-5°C and add saturated K 2 CO 3 solution. The organic phase was dried (Na 2 SO 4 ) was concentrated and purified by recrystallization to obtain ligand 1.44g (65%).mp100.0-101.3°C; [α] D 20 =+23.5(c, 1.00, CHCl 3 ); FTIR (KBr) 3333, 2972, 1606, 1523, 1357, 1197, 861cm -1 ; 1 HNMR (300MHz, CDCl 3 )δ8.19 (d, J=8.8Hz, 2H), 7.60 (d, J=8.4Hz, 2H), 4.59 (d, J=9.9Hz, 1H), 3.34 (dd, J=3.0Hz, and9. 9Hz, 1H), 3.21(dd, J=6.5Hz, and 10Hz, 1H), 2....
Embodiment 3
[0037] Preparation of (1R, 2R)-3-tert-butyldimethylsilyloxy-2-N, N-dimethylamino-1-p-nitrophenyl-1-propanol:
[0038] (1R,2R)-2-N,N-Dimethylamino-3-p-nitrophenyl-1,3-propanediol (1.946 g, 8.1 mmol) was dissolved in CH 2 Cl 2 (30mL), TBDMSCl (1.28g, 5.3mmol) and imidazole (1.4g, 20.6mmol) mixture was added at 0°C and stirred overnight to obtain product 2.72g. FTIR (KBr) 3344, 2954, 1606, 1525, 1349cm -1 ; 1 HNMR (300MHz, CDCl 3 )δ8.25-8.20 (d, J=8.5Hz, 2H), 7.6-7.55 (d, J=8.5Hz, 2H), 4.65 (d, J=9.7Hz, 1H), 3.77-3.6 (dd, J =11.3Hz, 2.7Hz 1H), 3.5-3.45(dd, J=11.3Hz, 6.0Hz 1H), 2.50(m, 7H), 0.90(s, 9H), 0.01(s, 6H); 13 CNMR (75MHz, CDCl 3 )δ150.2, 147.4, 128.0, 123.3, 69.0, 57.1, 41.6, 25.7, 17.9, -5.9; MS (EI) m / e297 (M+-57, 0.3), 209 (8.2), 202 (100).Anal .calcd.for C 17 h 30 N 2 o 4 Si: C, 57.60; H, 8.53; N, 7.90. Found: C, 57.82; H, 8.18; N, 7.77.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com