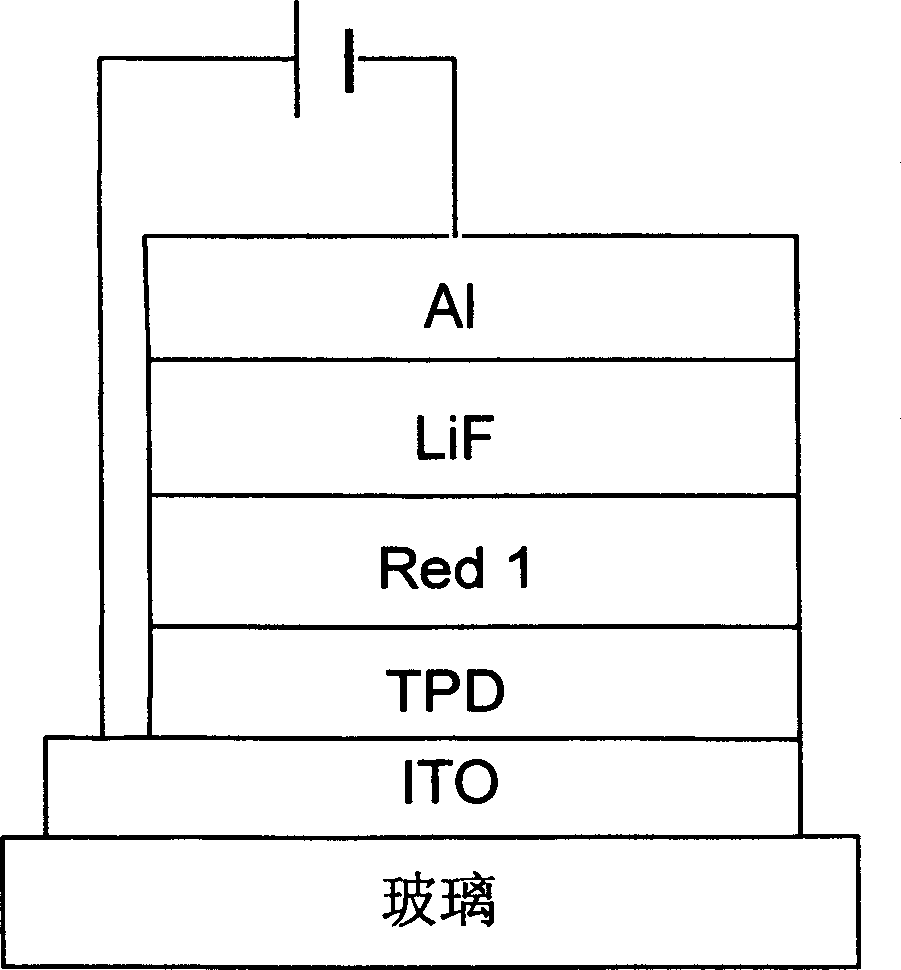
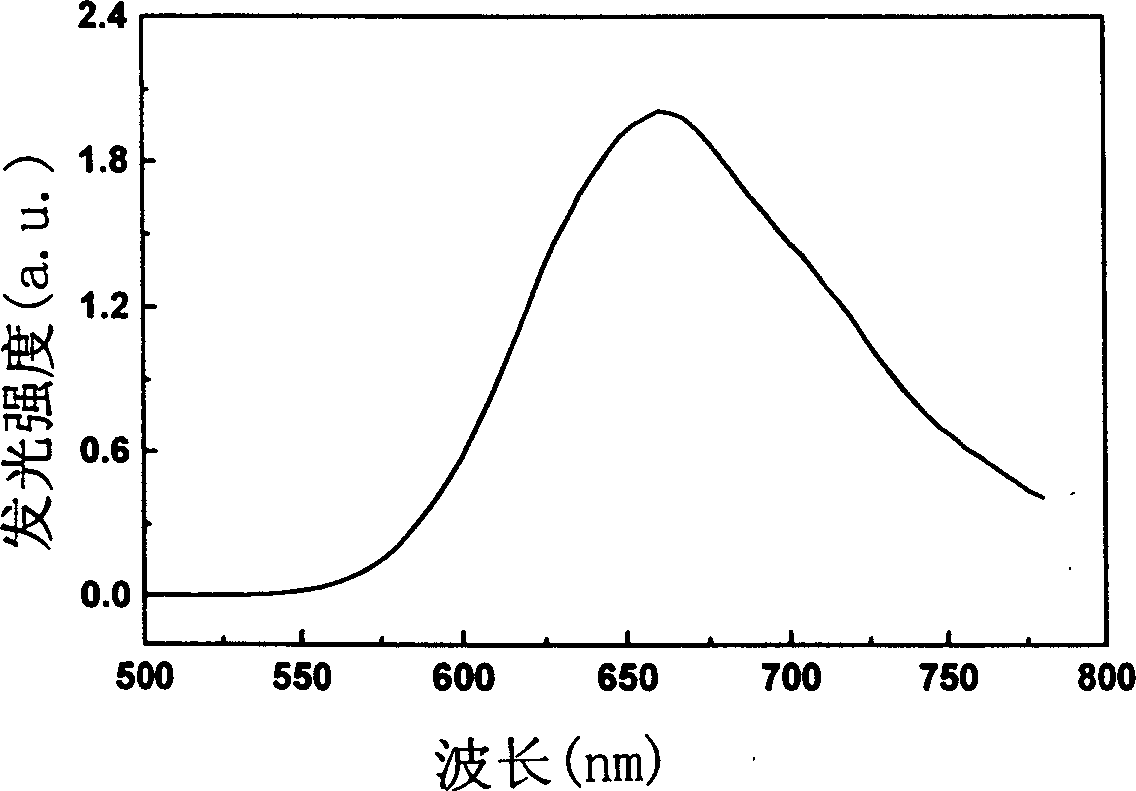
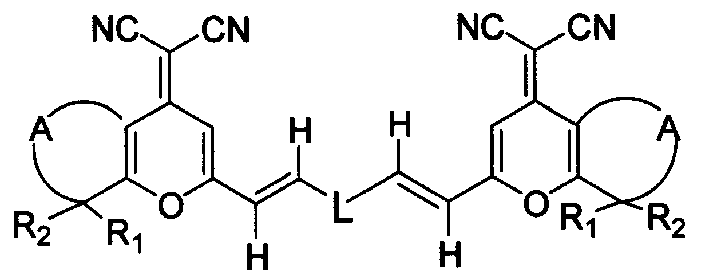
Red fluorescence dyestuff and its synthesizing method and usage
A technology of red fluorescence and synthesis method, which is applied in the field of fluorescent dyes with red light emission and its synthesis, can solve the problems of reducing reaction yield, increasing cost, raw material consumption, etc., and achieves high yield, long excited state life, and reaction The effect of fewer steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: N, N-bis-[4-[2-(4-dicyanomethenyl)-8-methyl-5,6,7,8-tetrahydro-4H-1-benzopyran ]vinyl]phenylaniline
[0048] Add 0.56g (2.5mmol) 4-dicyanomethenyl-2,8-dimethyl-5,6,7,8-tetrahydro-4H-1-benzopyran to a round bottom flask, 0.30g (1 mmol) N, N-bis-(4-formylphenyl) aniline, 15 ml acetonitrile, 0.40 ml hexahydropyridine, heated to reflux for 24 hours. The solvent was removed by distillation, cooled, and the solid was rinsed with acetonitrile for several times, and dried to obtain 0.52 g of the product with a yield of 72%.
[0049] NMR 1 H NMR (CDCl 3 )δ (ppm): 1.37 (d, 6H), 1.5-2.1 (m, 8H), 2.89 (m, 6H), 6.52 (d, 2H), 6.70 (s, 2H), 7.00-7.40 (m, 15H )
[0050] Elemental analysis calculated value (C 38 h 39 N 5 o 2 ): C, 80.31; H, 5.48; N, 9.76
[0051] Found: C, 80.13; H, 5.85; N, 9.44
[0052] Mass spectrometry (MS + ): 717 (M + )
Embodiment 2
[0053] Example 2: N, N-bis-[4-[2-(4-dicyanomethenyl)-8-methyl-5,6,7,8-tetrahydro-4H-1-benzopyran ]vinyl]phenyl-4-methylaniline
[0054] Add 0.56g (2.5mmol) 4-dicyanomethenyl-2,8-dimethyl-5,6,7,8-tetrahydro-4H-1-benzopyran to a round bottom flask, 0.32g (1mmol) N, N-bis-(4-formylphenyl)-4-methylaniline, 15ml acetonitrile, 0.40ml catalyst (its preparation method is: 5ml hexahydropyridine is dissolved in 15ml acetic acid), heated to reflux for 24 Hour. The solvent was removed by distillation, cooled, and the solid was rinsed with acetonitrile for several times, and dried to obtain 0.48 g of the product with a yield of 65%.
[0055] NMR 1 H NMR (CDCl 3 )δ (ppm): 1.38 (d, 6H), 1.5-2.1 (m, 8H), 2.64 (s, 3H), 2.87 (m, 6H), 6.52 (d, 2H), 6.70 (s, 2H), 6.80-7.40(m, 14H)
[0056] Elemental analysis calculated value (C 49 h 41 N 5 o 2 ): C, 80.41; H, 5.65; N, 9.57
[0057] Found: C, 80.32; H, 5.85; N, 9.41
[0058] Mass spectrometry (MS + ): 731 (M + )
Embodiment 3
[0059] Example 3: N, N-bis-[4-[2-(4-dicyanomethenyl)-8-methyl-5,6,7,8-tetrahydro-4H-1-benzopyran ]vinyl]phenyl-4-methoxyaniline
[0060] In a round bottom flask, add 0.68g (3mmol) 4-dicyanomethenyl-2,8-dimethyl-5,6,7,8-tetrahydro-4H-1-benzopyran, 0.33g ( 1mmol) N, N-bis-(4-formylphenyl)-4-methoxyaniline, 15ml acetonitrile, 0.40ml catalyst (its preparation method is: 5ml hexahydropyridine is dissolved in 15ml acetic acid), heated to reflux for 24 Hour. The solvent was removed by distillation, cooled, and the solid was rinsed with acetonitrile for several times, and dried to obtain 0.53 g of the product with a yield of 71%.
[0061] NMR 1 H NMR (CDCl 3 )δ (ppm): 1.38 (d, 6H), 1.5-2.1 (m, 8H), 2.87 (m, 6H), 4.41 (s, 3H), 6.52 (d, 2H), 6.70 (s, 2H), 6.80-7.40(m, 14H)
[0062] Elemental analysis calculated value (C 49 h 41 N 5 o 3 ): C, 78.69; H, 5.53; N, 9.36
[0063] Found: C, 78.13; H, 5.85; N, 9.24
[0064] Mass spectrometry (MS + ): 747 (M + )
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com