Infection of eukaryotic cells with viruses in vitro
A technology for eukaryotic cells and infecting cells, applied to viruses, antiviral agents, cells modified by introducing foreign genetic material, etc. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
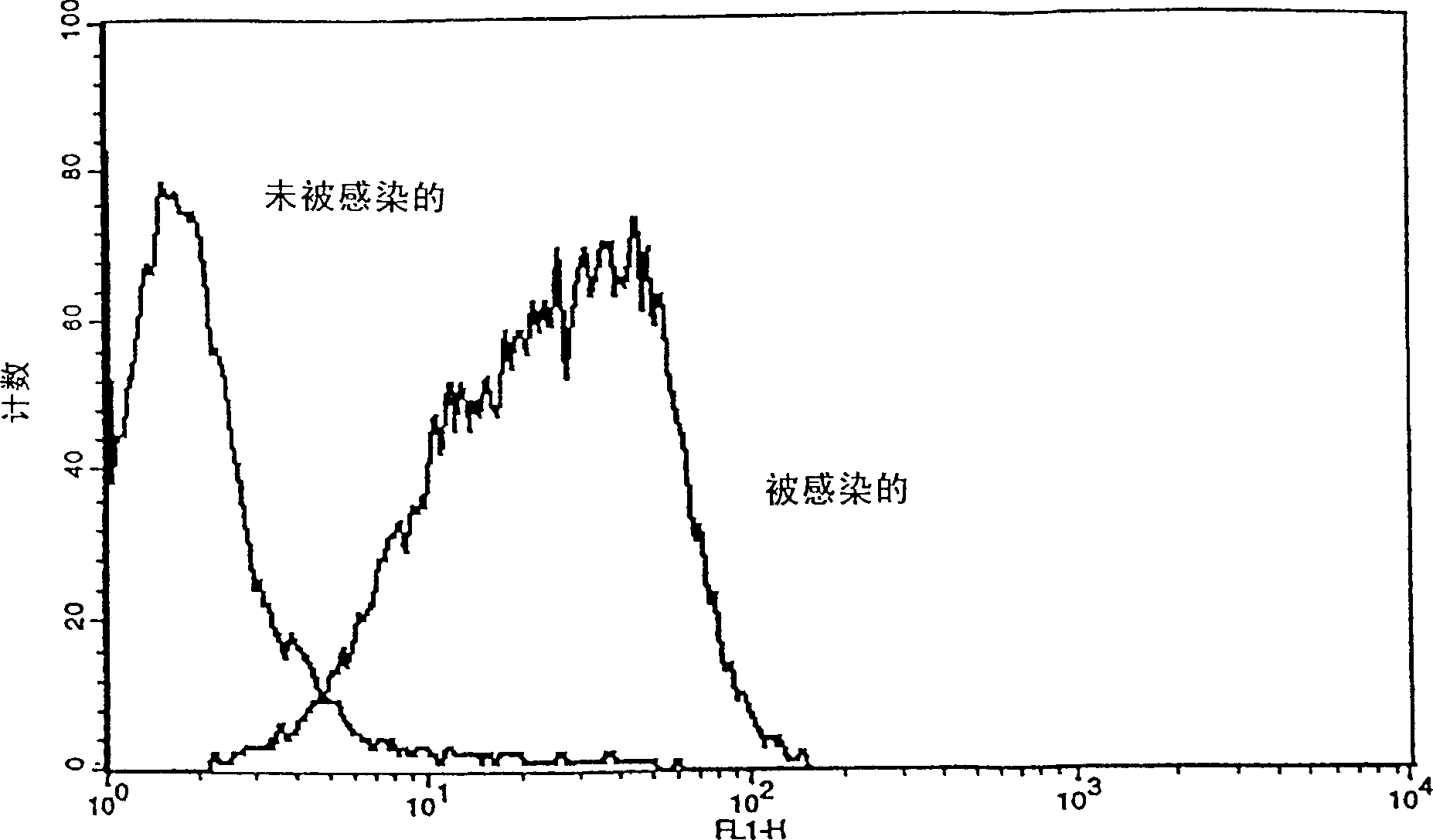
[0044] This example demonstrates the effective and stable infection of HepG2 cell line by HBV. The proof of HBV infection is supported by the following tests: the presence of HbsAg in the supernatant (1) and bound to the cells (2), (3), and (4); the transformation effect of HBV (5); dextran Overgrowth in the combined treatment of methasone and insulin (6); long-term insulation (7). (1) The presence of HBsAg in the cell culture supernatant
[0045]In order to detect HBsAg in the supernatant or on the cell surface, the AXsYM (Abbott Laboratories) routine test is usually used to detect the HBV surface antigen. In order to maintain a convenient concentration of protein in the sample to obtain accurate results, the following procedure is usually considered: usually the cell culture supernatant from the mock infection culture and HBV infection culture is mixed with AUSAB-negative, HBV-negative human serum. A final concentration of 50-90% serum is reached in the analyzed sample. The resu...
Embodiment 2
[0055] This example demonstrates the effective and stable infection of Huh-7 cell line by HBV. Proof of HBV infection is supported by long-term insulation.
[0056] According to the method of the present invention, Huh-7 cells are infected with low-titer HBV. The cells can then be kept as a monolayer for up to 35 days (Table 4a). During the entire incubation period, only about 75-80% of the volume of the complete medium is replaced every 5-7 days.
[0057] Table 4a
Embodiment 3
[0059] This example demonstrates the effective and stable infection of HepG2 cell line by HCV. The proof of HCV infection is supported by RNA detection (COBAS AmpliGor) in the supernatant and cytopathic effects.
[0060] To detect HCV, Roche's COBAS Amplicor HCV Monitor test is routinely used. In order to maintain a convenient concentration of protein in the sample to obtain accurate results, the following procedure is usually considered: usually the cell culture supernatant from the simulant infection culture and the HCV infection culture is mixed with HCV-negative human serum, the sample that will usually be analyzed In the final concentration of 50-90% serum. HCV negative serum was used as a negative control; as for the positive control, human plasma or human plasma from HCV infected patients with known HCV viremia was treated in a similar manner to samples from mock-infected cells and HCV-infected cell culture medium serum.
[0061] The results are given in the form of an inde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com