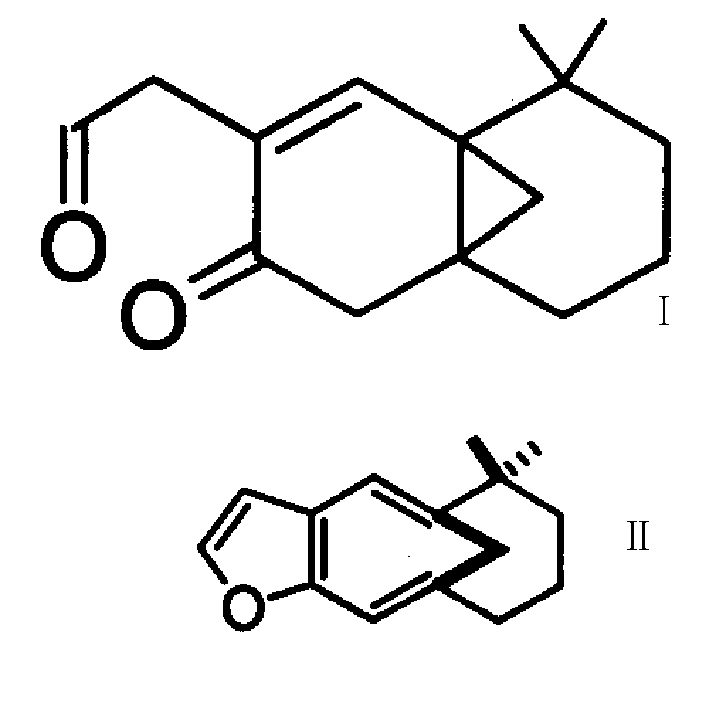
3-substituted 5,6,7,8 tetrahydro-4a,8a method-2-keto-naphthalene, synthesis method and use thereof
A synthetic method and a fork technology, applied in 3 to replace 5, can solve problems such as poor synthetic strategy, low yield, lengthy synthetic route, etc., and achieve the effects of good reaction yield, improved yield, and cheap and easy-to-obtain raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthesis of embodiment 1 compound 2
[0035] 2.5ml of diisopropylamine was dissolved in 12ml of tetrahydrofuran, cooled to -78°C, and 7.0ml of 2.4M (16.8mmol) nBuLi was added dropwise. Stop cooling after addition, slowly warm up to 0°C, and stir for 30 minutes. spare.
[0036] A solution of 3.7 g (15.6 mmol) of compound 1 in 5 ml of tetrahydrofuran was slowly added dropwise thereto at -78°C. After stirring and reacting at -78°C for 2 hours, 1.5ml (17mmol) of allyl bromide was added dropwise, and after the addition was completed, the mixture was naturally warmed to room temperature and reacted for 8 hours. Slowly add 0.5N dilute hydrochloric acid solution dropwise to the reaction liquid under ice bath to quench the reaction. Extracted three times with ether. Combine the organic phases, wash with water, wash with saturated NaCl solution, Na 2 SO 4 dry. The solvent was removed, and 2.20 g (63%) 2 and 320 mg (9%) 2a were obtained by passing...
Embodiment 2
[0040] The synthesis of embodiment 2 compound 3
[0041] 135mg (2eq.) LiAlH 4 Suspended in 8ml of THF, slowly added dropwise a solution of 489mg (1.77mmol) of compound 2 in 2ml of THF at room temperature. The reaction was completed for 1.5hr. The reaction was quenched by adding solid sodium sulfate moistened with water to the reaction solution. Filter and wash the filter residue with tetrahydrofuran. The washing liquid and the filtrate were combined, the solvent was removed, and the residue was passed through the column to obtain 335 mg (76%) of colorless oily substance 3. Compound 3: C 16 h 26 o 2 (250.39) IR (film, cm -1 ): ν3368, 3075, 1640. 1 H-NMR (CDCl 3 ): δ5.92(1H, dd, J=9.8Hz and 2.2Hz, 1-H), 5.82(1H, m, 13-H),
[0042] 5.03 (2H, m, 14-H), 3.64 (1H, d, J=11.7Hz, 11-Ha), 3.47 (1H, d, J
[0043] = 11.8 Hz, 11-Hb), 1.03, 0.82 (2 x 3H, s, 5, 5-dimethyl). EIMS: m / e 250 (M), 215 (100%).
Embodiment 3
[0044] The synthesis of embodiment 3 compound 4
[0045] 108mg (0.43mmol) of compound 3 was dissolved in 9ml of n-hexane. Add 108mg of DDQ and react at room temperature for 30 hours. Filter, wash with 2N sodium hydroxide, wash with water, wash with saturated sodium chloride, and dry over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the residue was separated by column chromatography to obtain 67 mg (70% deduction of unreacted raw materials) of white solid 4, and 11 mg of compound 6 was recovered (90% conversion). Compound 4: C 16 h 24 o 2 (248.37) m.p.92-93℃IR (film, cm -1 ): ν3429, 3065, 1650, 1618. 1 H-NMR (CDCl 3 ): δ6.03(1H, s, 1-H), 5.80(1H, m, 13-H), 5.05(2H, m, 14-H),
[0046] 4.15(1H,dd,J=10.9Hz and 1.5Hz,11-Ha), 3.94(1H,d,J=10.9
[0047] Hz, 11-Hb), 0.95, 0.91 (2×3H, s, 5,5-dimethyl). EIMS: m / e 249 (M+1), 107 (100%). Elemental analysis: Calculated value. (%): C77.38, H9.74 Measured value (%): C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com