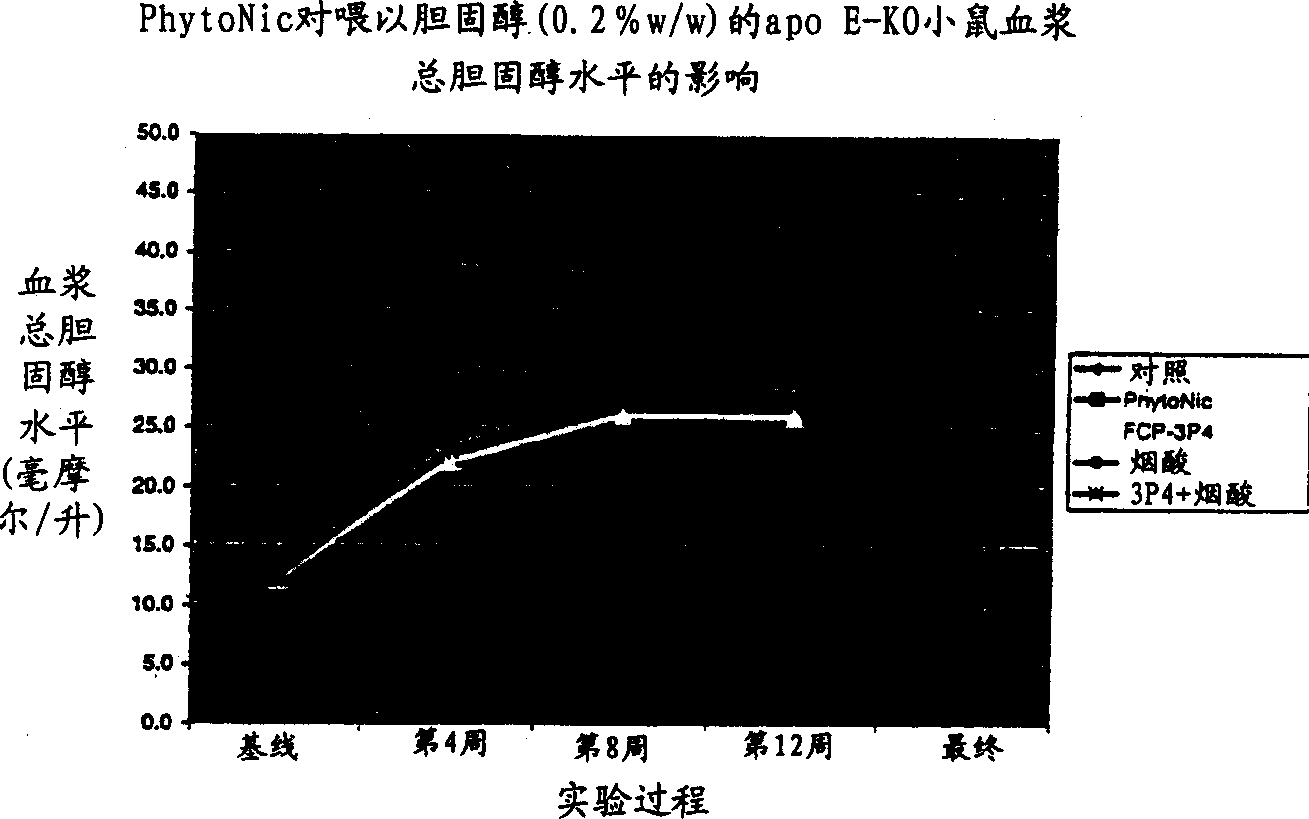
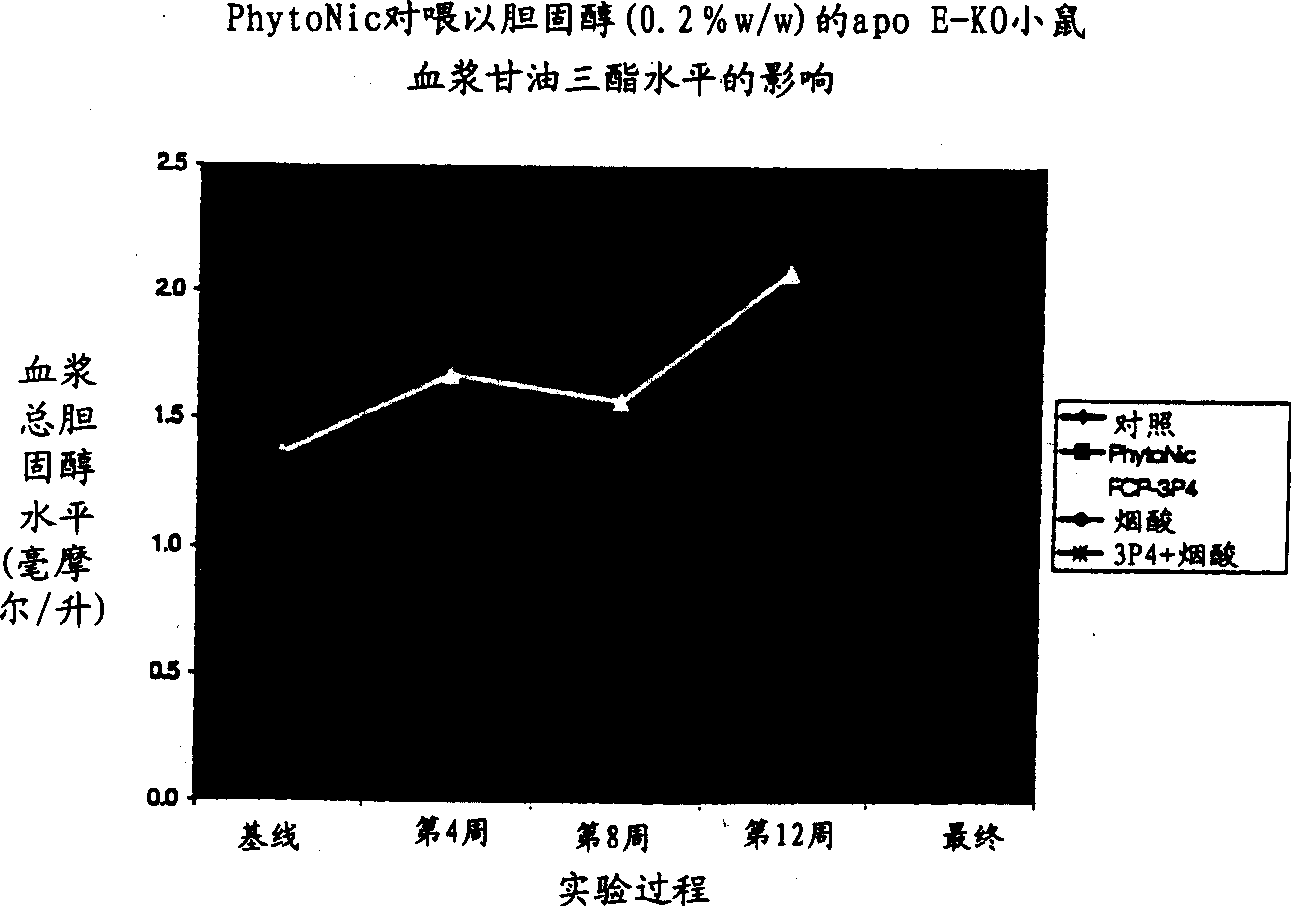
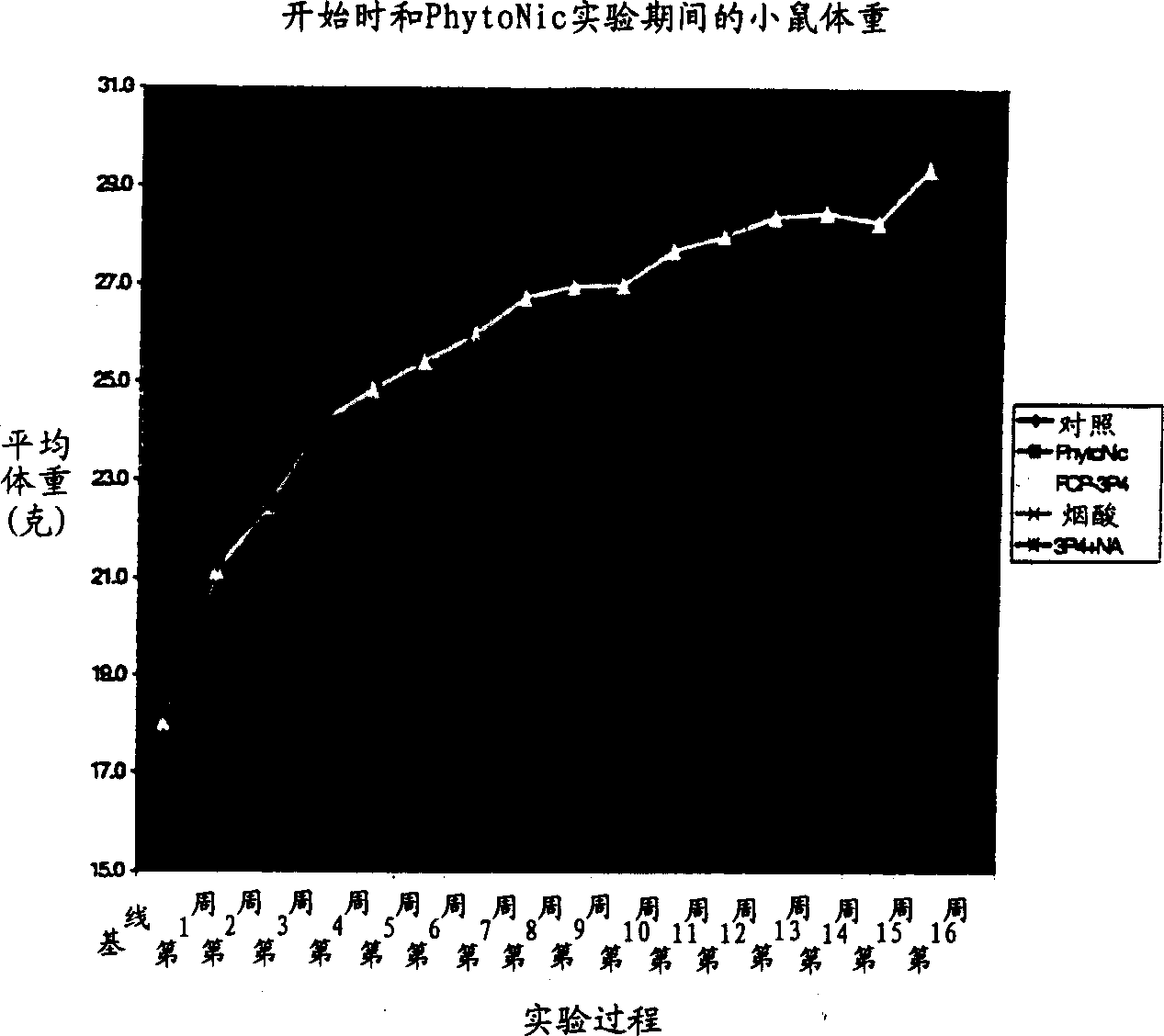
Aromatic and heterocyclic derivatives of phytosterols and/or phytosanols for use in treating or preventing cardiovascular disease
A technology of phytostanols and phytosterols, which is applied in the field of treatment and prevention of cardiovascular diseases and other diseases, can solve the problems of low solubility of phytosterols and complex administration of phytosterols, and achieve the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Example 1 Preparation of Niacin Stanol Ester
[0129] Nicotinyl chloride (6 g) was suspended in chloroform (150 mL) and a stanol mixture (11 g, campesterol 36.4%; sitostanol 62.3%). The mixture was stirred at room temperature, and anhydrous pyridine (6 ml) was added dropwise to the above-prepared mixture. Stirring was continued for 3 hours. After the addition was complete, the mixture was concentrated, the solvent was removed, and acetone (100 mL) was added to the remaining residue. The resulting white precipitate was collected and recrystallized from acetone:chloroform (2:1). 10 g of white crystals are obtained.
Embodiment 2
[0130] Example 2 Preparation of Niacin Stanol Hydrochloride
[0131] The nicotinic acid stanol ester (2 g) prepared above was dissolved in anhydrous ether (20 ml), and 36% HCl (1 ml) was added thereto at room temperature and stirred. The white precipitate was filtered off, washed with diethyl ether (10 mL), and dried to obtain nicotinic acid stanol hydrochloride (2 g) as a white powder.
Embodiment 3
[0132] Example 3 Preparation of stanol nicotinyloxyacetate
[0133] Step a. Synthesis of stanol monochloroacetate
[0134] A stanol-containing mixture (4 g, campestanol (36.4%) and stanol (62.3%)) in monochloroacetic acid (10 mL) was stirred with heating at 120° C. for 3 hours. After cooling, 50 mL of water was added to the reaction mixture, and the precipitate was collected, washed with 10 mL of water, and dried to give a white solid (4.5 g).
[0135] Step b. Synthesis of stanol nicotinyloxyacetate. Anhydrous DMF (10 ml) containing sodium nicotinate (0.5 g) was added to the stanol chloroacetate (1.5 g) prepared above, and the mixture was heated and stirred at 140° C. for 1 hour. After cooling to room temperature, the mixture was poured into 100 mL of water and an off-white solid was collected, dried and weighed (1.8 g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com