Kjeldahl comprehensive syndrome fetal molecule dingnosis reagent box and application
A molecular diagnosis and syndrome technology, which is applied in the field of biomedical technology and can solve problems such as inability to perform prenatal villus or amniotic fluid cell chromosome examinations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1: Preparation of Fetal Molecular Diagnosis Kit for Klinefelter Syndrome
[0082] Klinefelter Syndrome Fetal Molecular Diagnosis Kit includes DNA Extraction Solution A, DNA Extraction Solution B, Proteinase K, PCR Reaction Solution, PCR Specific Primer 1, PCR Specific Primer 2, Taq Enzyme, Standard I, Standard II and Positive Control goods, negative control substances, of which:
[0083] DNA extraction solution A is cell lysate, including: EDTA, sodium dodecyl sulfate (SDS), DNase-free RNase lysis buffer.
[0084] DNA extraction solution B is the precipitation solution, usually NH 4 Ac salts.
[0085] The PCR reaction solution contained 2.5 μl of 10×PCR buffer, 2 μl of MgCL, 0.5 μl of dNTP and 17.75 μl of deionized water.
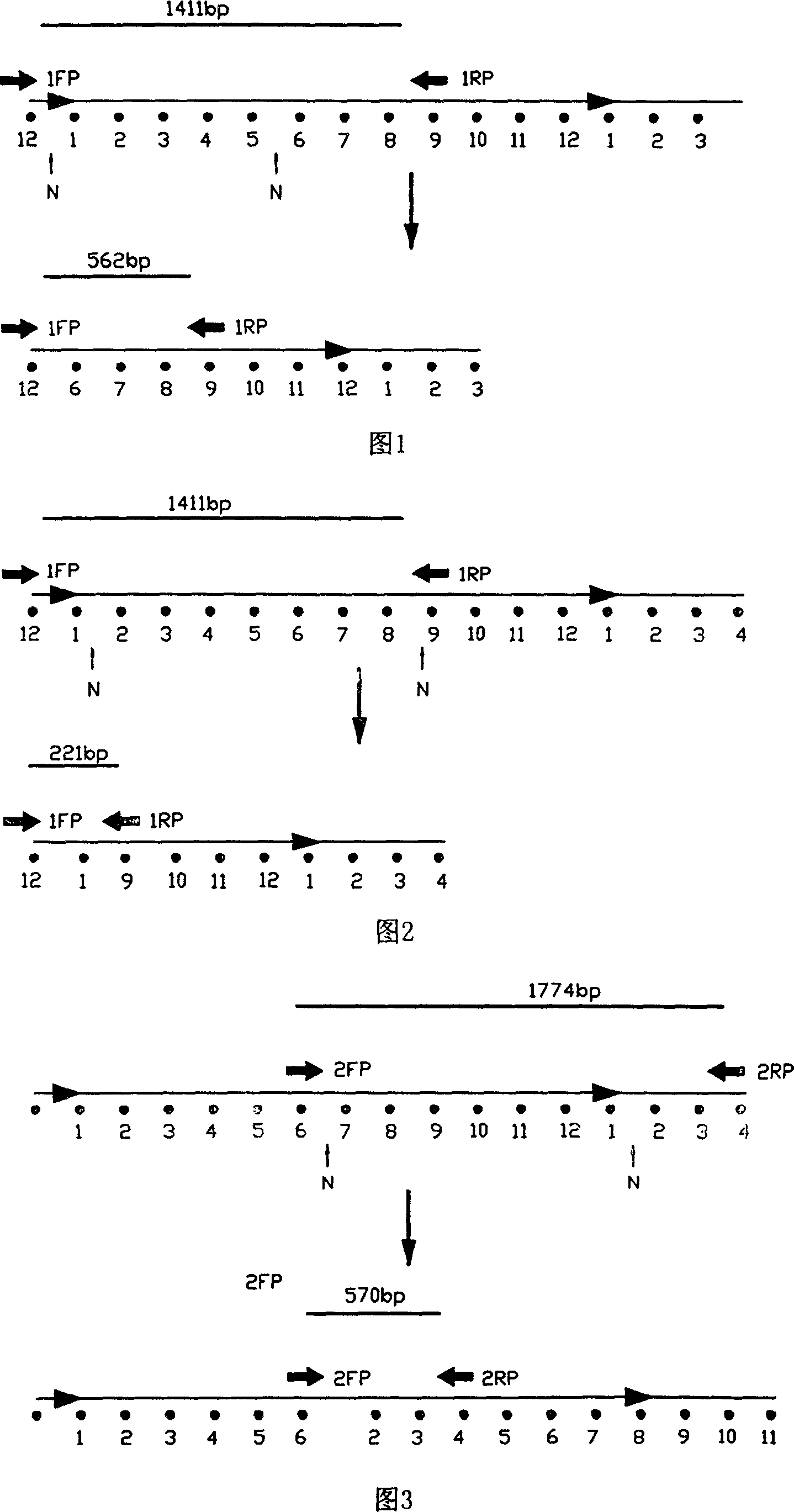
[0086] PCR-specific primer 1 is: sense strand 5′-aag cct ttt cct tta tct tca ca-3′,
[0087] Antisense strand 5′-ctg aga atg ctt ccg ttt gcc-3′,
[0088] PCR specific primer 2 is: sense strand 5′-att ctc aga aag ttt tct gc-3′,
[0089] ...
Embodiment 2
[0117] Example 2: Detection of molecular markers in peripheral blood of parents with Klinefelter syndrome fetal molecular diagnostic kit
[0118] (1) Sample DNA preparation
[0119] 1. Take 1ml of peripheral blood, add sodium citrate for anticoagulation, and then take out 200μl into a 1.5ml sterile EP tube;
[0120] 2. Add 400 μl of DNA extraction solution A to the EP tube, then add 3 μl of proteinase K and mix well, place in a 55°C water bath for 5-10 minutes or longer, mix up and down several times during the process, and adjust the digestion time according to the degradation degree of the sample;
[0121] 3. Add 600 μl chloroform and mix well, not too vigorously, to ensure the integrity of DNA;
[0122] 4. Centrifuge at room temperature for 2 minutes at 10,000 rpm on a desktop high-speed centrifuge;
[0123] 5. Divide into three layers after centrifugation. Genomic DNA is in the upper layer. Take 500 μl of the supernatant and transfer it to a 1.5ml sterile EP tube. The mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com