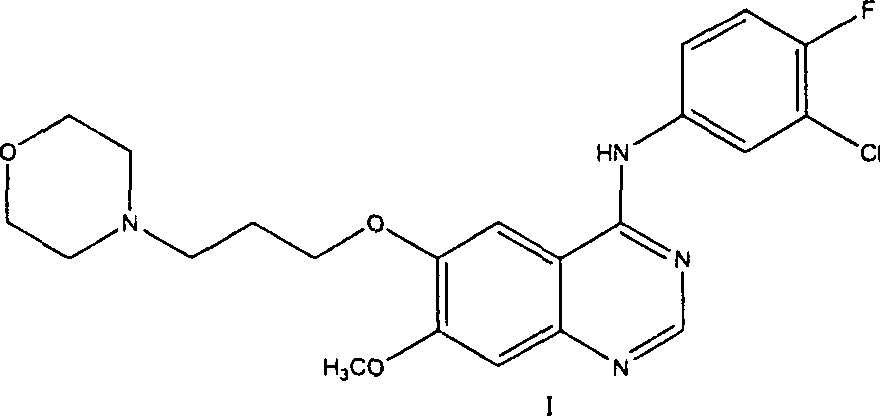
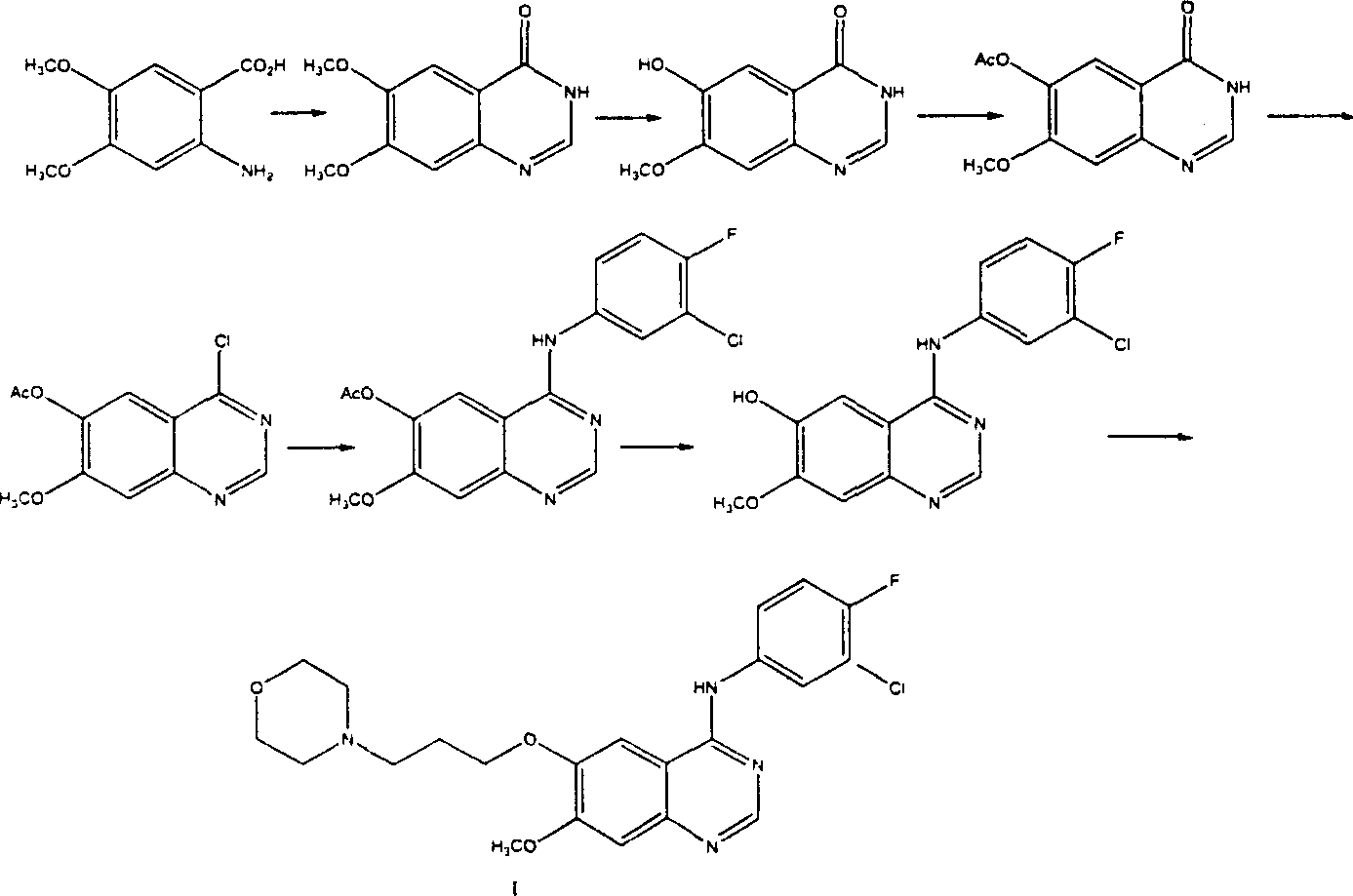
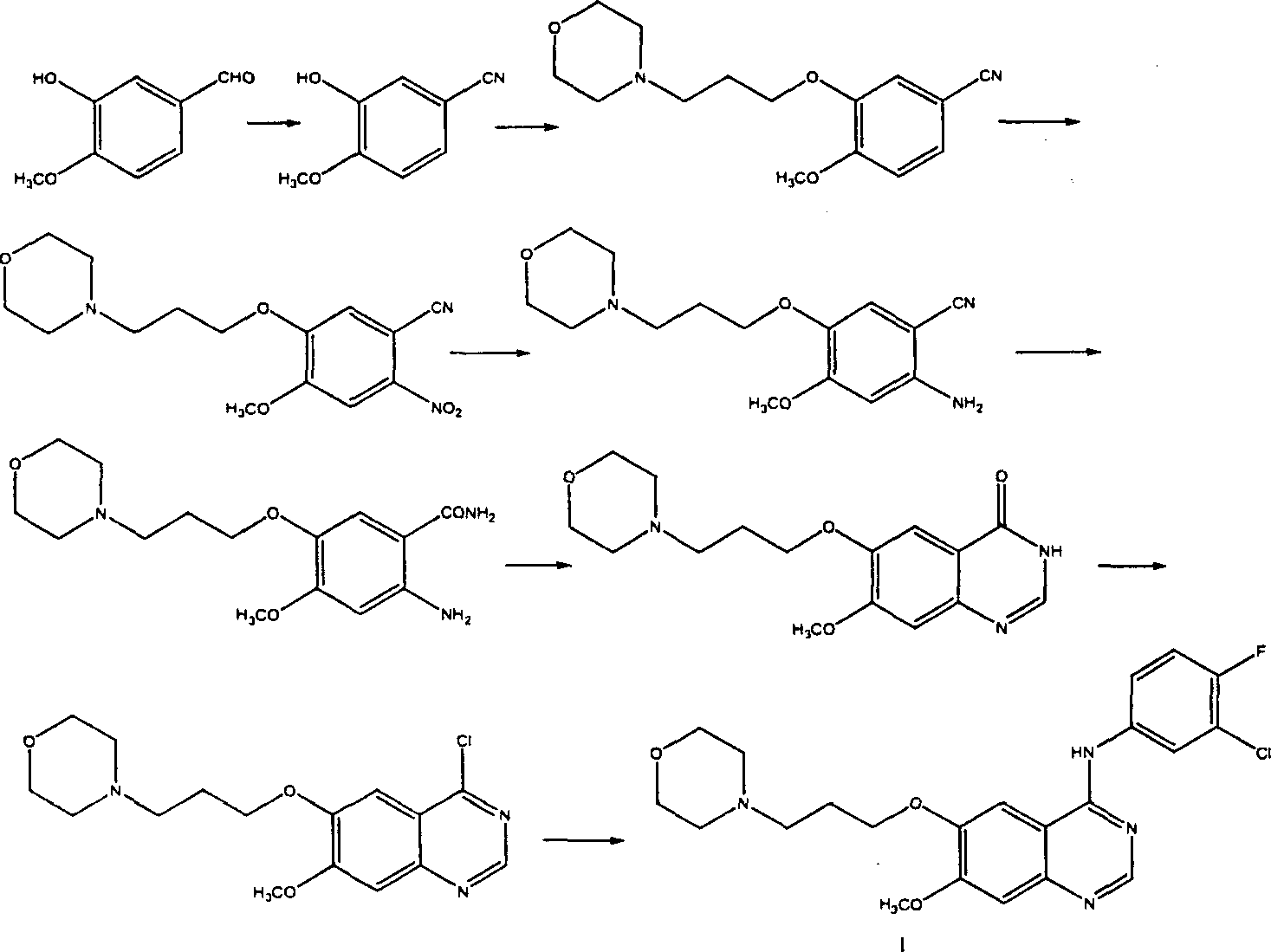
Preparation method of 4-(3-chlor-4-fluorobenzeneamidocyanogen)-7-methoxy-6-(3-morpholine oxypropyl)quinazoline
A technology of fluorophenylamine group and morpholine propoxy group, which is applied in the field of preparation of 4--7-methoxy-6-quinazoline, can solve problems such as high price and reduce reaction steps and reaction routes concise effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1,3, the preparation of 4-dimethoxy-6-nitrobenzoic acid (III):
[0028] Add 182g of 3,4-dimethoxybenzoic acid (II) to 1000mL of concentrated nitric acid (d, 1.42) at 0-5°C in portions within 1 hour. In crushed ice, the yellow solid was filtered, washed with water, and recrystallized from ethanol to obtain 185 g of yellow needle crystals, mp 190-1°C, yield 81.5%.
Embodiment 2
[0029] Embodiment 2, the preparation of 3-hydroxyl-4-methoxy-6-aminobenzoic acid (V):
[0030]Add 185g of 3,4-dimethoxy-6-nitrobenzoic acid (III) to 1250mL of 10% potassium hydroxide aqueous solution, heat and stir at 20-100°C for 2-4 hours, then, within 1 hour Add 460g of sodium hydrochloride in portions, keep the reaction at 40-70°C for 1 hour, acidify with concentrated hydrochloric acid under ice bath cooling, separate the white precipitate, and crystallize with dilute ethanol to obtain 3-hydroxy-4-methoxy-6-aminobenzoic acid (V), colorless needle crystals, 122.5g, mp214~5°C, yield 82%.
Embodiment 3
[0031] Embodiment 3, the preparation of 6-hydroxyl-7-methoxy-3,4-dihydroquinazolin-4-one (VI)
[0032] 122.5g of 3-hydroxy-4-methoxy-6-aminobenzoic acid (V), mixed with 380mL of formamide, heated to 60-210°C, stirred for 12 hours, cooled, poured into ice water, filtered, After washing with water, 116.7 g of needle crystals of 6-hydroxy-7-methoxy-3,4-dihydroquinazolin-4-one (VI) were obtained, mp>200°C, yield 90.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com