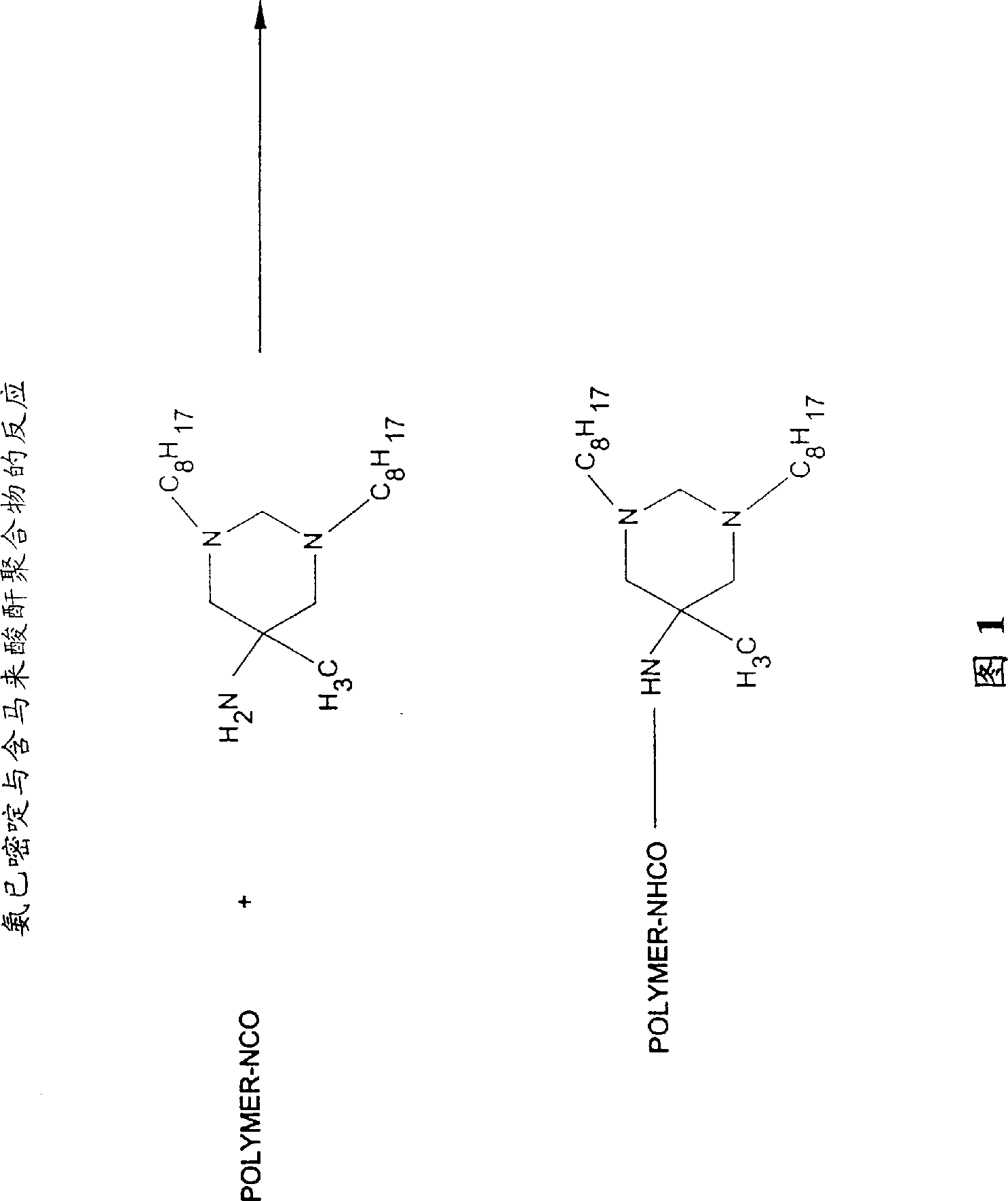
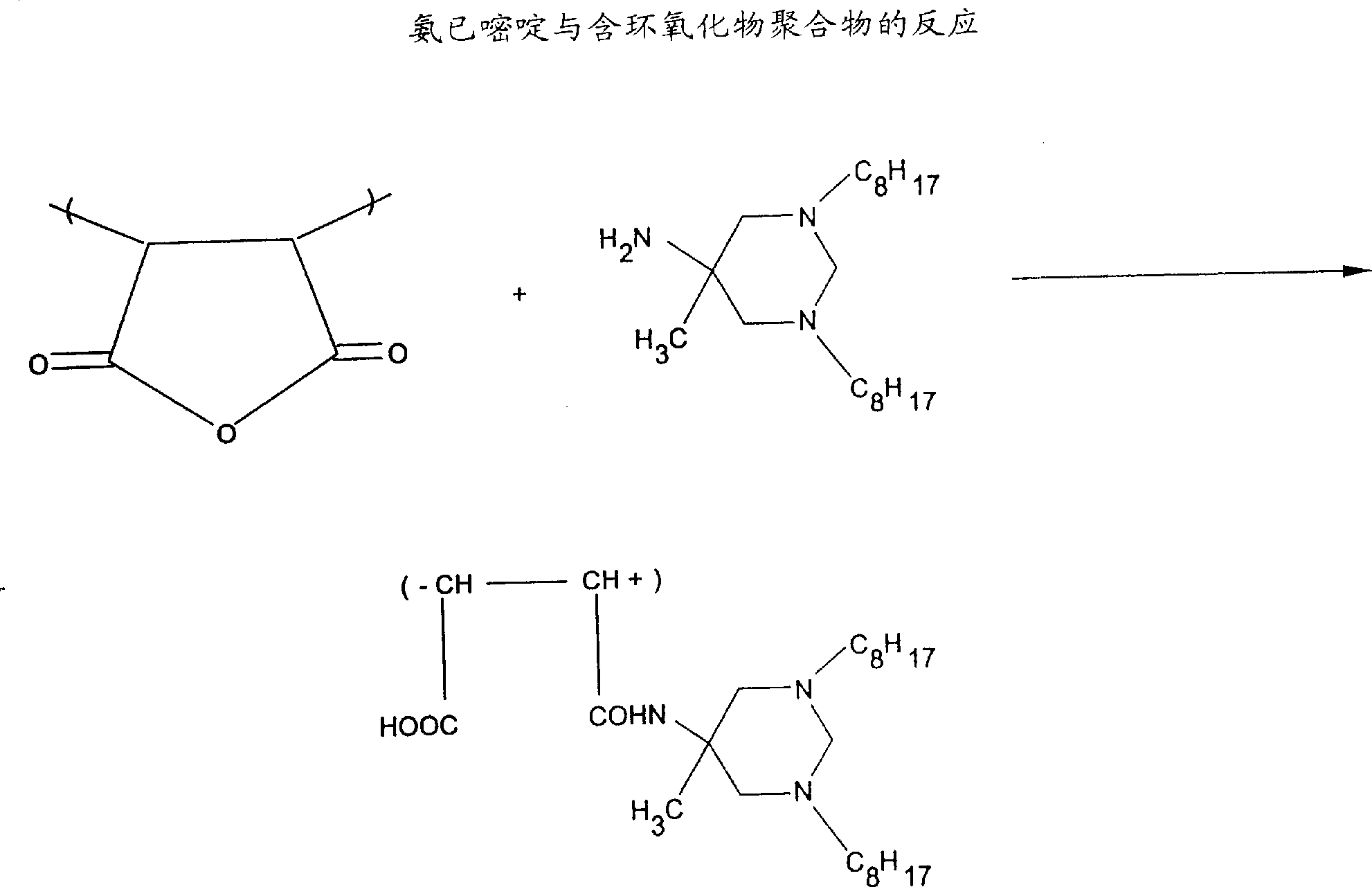
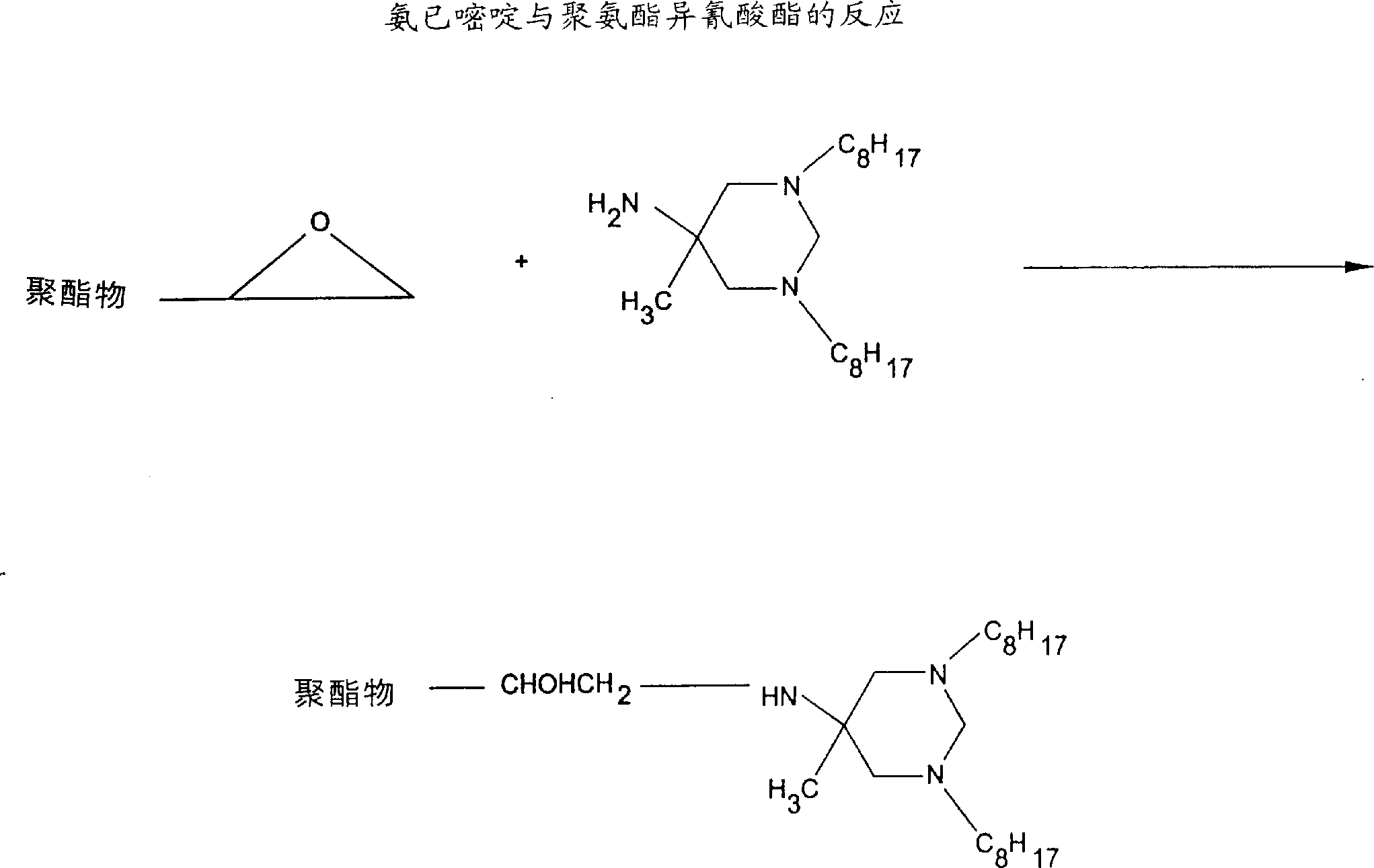
Biostatic coatings for reduction and prevention of bacterial adhesion
A biological and functional technology, applied in the field of biofunctional structural composites, to achieve the effect of reducing the coefficient of friction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0089] Biofunctional structure composition of the present invention
[0090] 2 grams of polyurethane polyisocyanate prepolymer (NORDOTAdhesive 34D-2, Synthetic Surfaces, Inc.) prepared by reacting 2 molar excess of diphenylmethane diisocyanate (MDI) with ricinoleic acid ester polyols was mixed with 250 mg of carboxycin (ANGUS Chemical) was mixed in 35 g methyl ethyl ketone. The reaction was monitored by gas chromatography. There was significant consumption of methamphetamine within 8 hours. Infrared analysis shows that at about 2400cm -1 The isocyanate peak disappears. 10 g of tetrahydrofuran, 10 g of N-methylpyrrolidinedione, 30 g of diacetone alcohol, and 3 g of polyvinylpyrrolidinedione (KOLLIDONE 90F, BASF) were added to the solution. Soak the cleaned polyvinyl chloride catheter in the solution for 15 minutes, air dry it for 30 minutes, and then cure it at 80°C for 30 minutes.
[0091] The bacterial adhesion of Staphylococcus aureus on the catheter was detected by the...
Embodiment 3
[0093] Hydrophilic composition of the present invention
[0094] 2 grams of polyurethane polyisocyanate prepolymer (NORDOTAdhesive 34D-2, Synthetic Surfaces, Inc.) prepared by reacting 2 molar excess diphenylmethane diisocyanate (MDI) with ricinoleic acid ester polyol and 35 g of methyl ethyl ketone, 10 g Tetrahydrofuran, 10 g of N-methylpyrrolidinedione, 30 g of diacetone alcohol, and 3 g of polyvinylpyrrolidinedione (KOLLIDONE 90 F, BASF) were mixed. Apply the solution to a clean polyvinyl chloride sheet with a cotton swab. The sheet was air-dried for 30 minutes, and then aged at 80° C. for 30 minutes.
[0095] The bacterial adhesion of Staphylococcus aureus on the sheet was detected by the above-mentioned test method. Take a portion of TSB on the slide, and observed bacterial adhesion (>3×107cfu / ml); take a portion of the final PBS wash solution on the slide, no colony formation (<100 cfu / ml) was observed.
Embodiment 4
[0097] Composition of the invention
[0098] Vorite 3025 polyisocyanate prepolymer (2 g) (CasChem, Inc.) was mixed with methotrexate (0.25 g) in methyl ethyl ketone (35 g). The mixture was stirred vigorously overnight. Gas chromatographic analysis showed the disappearance of methamphetamine. Infrared analysis shows 2269cm -1 The isocyanate band disappears. Tetrahydrofuran (10 g), diacetone alcohol (30 g), N-methylpyrrolidinedione (10 g), polyvinylpyrrolidinedione (3 g; Kollidon 90 F), fluorinated alkyl alkoxy ester ( 0.1g; Flourad FC-171) and methyl ethyl ketone (14.65g), then the mixture was stirred evenly.
[0099]Apply the solution to a clean polyvinyl chloride (PVC) sheet with a cotton swab soaked in the solution. No adhesion of Staphylococcus aureus was found on the solution-coated sheets after the bacterial adhesion analysis. Take a portion of PBS washing solution on the slide, and no bacterial adhesion was found. No methexidine was found in the extracted medium. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com