Compound prepn. contg. Brufen arginine codeine
A technology of arginine codeine and arginine, which is applied in anti-inflammatory agents, pill delivery, pharmaceutical formulations, etc., can solve the problems of slow onset of action and gastrointestinal irritation, achieve quick onset of action, improve water solubility Sexuality and the effect of improving the absorption rate of the human body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
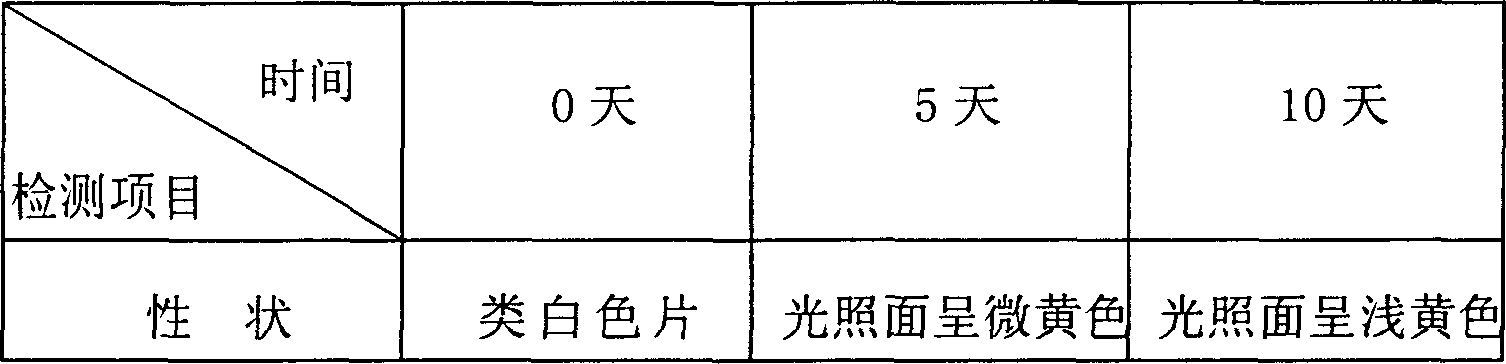
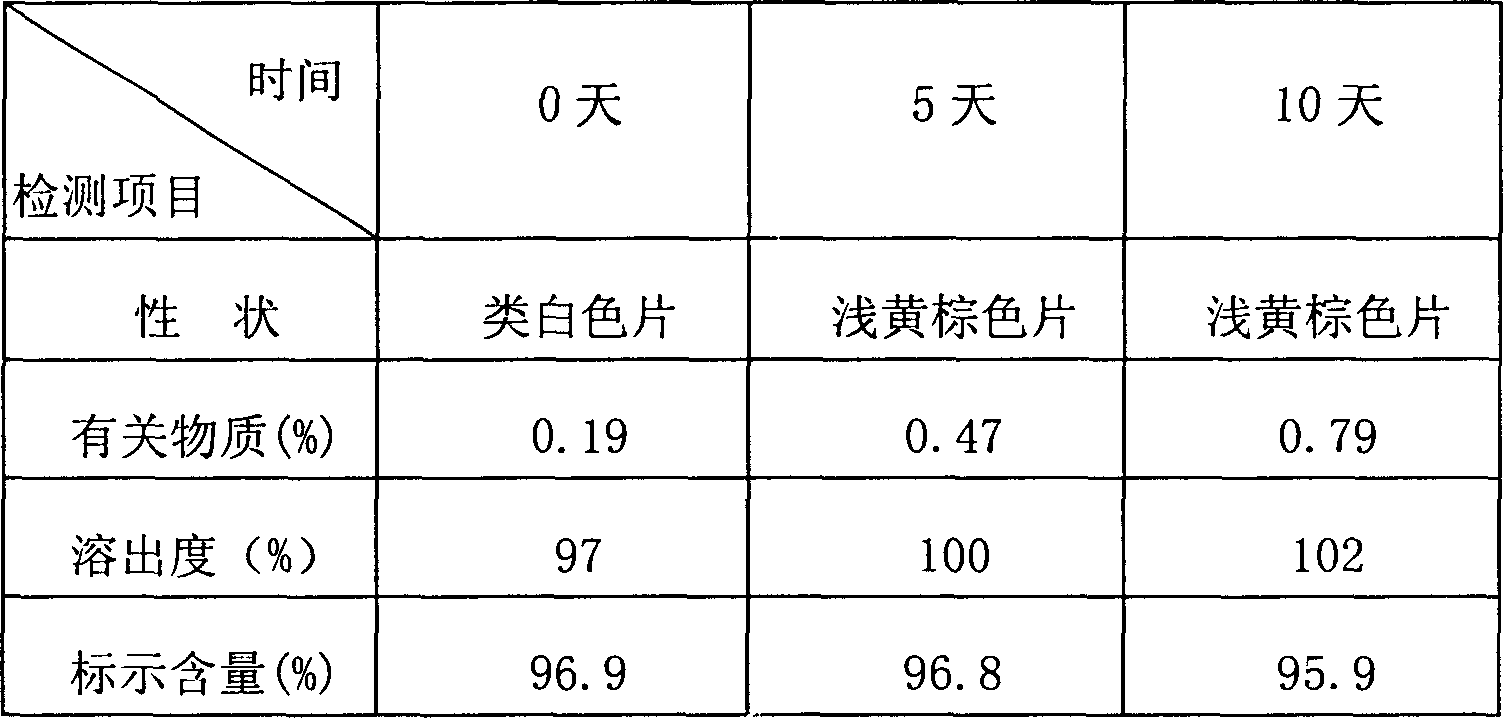
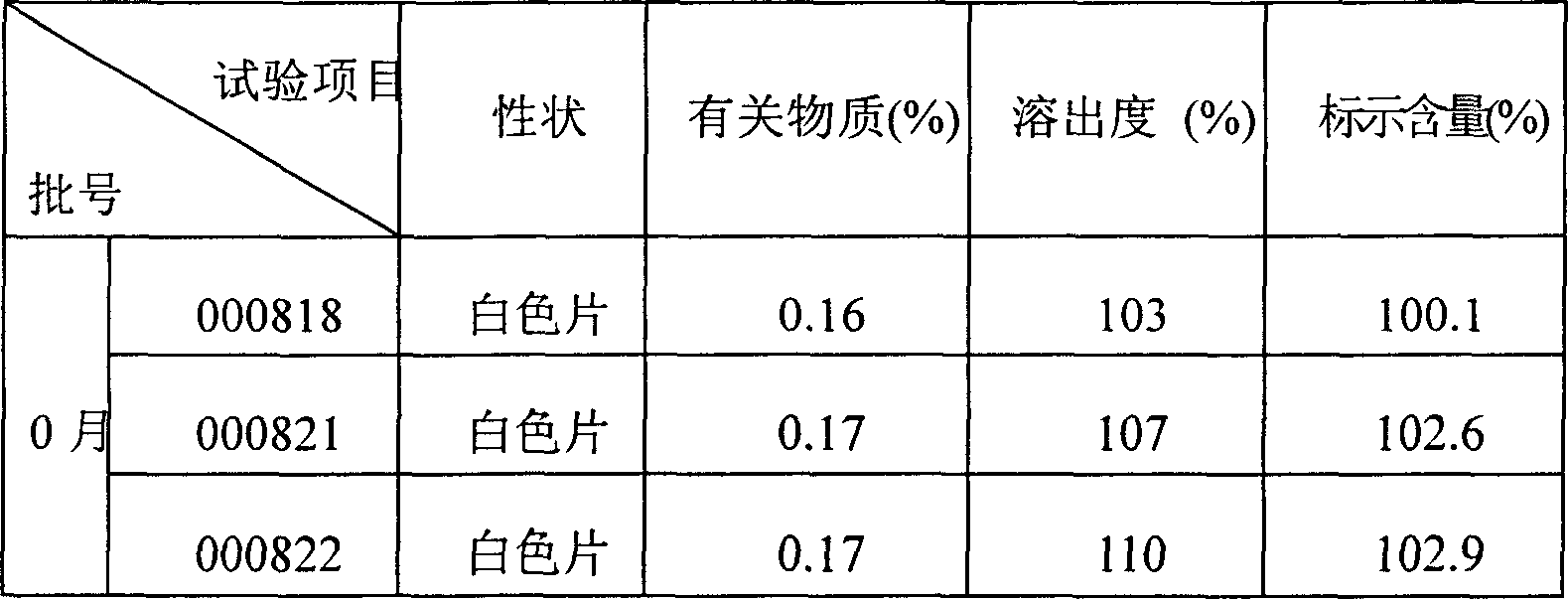
[0068] Embodiment 1: Preparation of ibuprofen arginine codeine compound tablet
[0069] Prescription (per 1000 tablets):
[0070] Ibuprofen Arginine 370g
[0071] Codeine Phosphate 12.5g
[0072] Lactose 37.5g
[0073] Starch 37.5g
[0074] Microcrystalline Cellulose 15g
[0075] 2% povidone aqueous solution 25ml
[0077] Magnesium stearate 0.9g
[0078] Process:
[0079] (1) Pass the raw materials and auxiliary materials through an 80-mesh sieve respectively;
[0080] (2) get ibuprofen arginine and codeine phosphate prescription quantity and mix with starch, then add lactose, microcrystalline cellulose and mix;
[0081] (3) Add 2% povidone aqueous solution to make soft material, granulate, dry at 70°C, granulate with 16 meshes, add talcum powder and magnesium stearate, mix evenly, and compress into tablets.
Embodiment 2
[0082] Embodiment 2: Preparation of ibuprofen arginine codeine compound capsule
[0083] Prescription (per 1000 capsules):
[0084] Ibuprofen Arginine 570g
[0085] Codeine Phosphate 12.5g
[0086] Hydroxypropyl ethyl cellulose 10g
[0087] Starch 5g
[0088] Micronized silica gel 5g
[0089] Process:
[0090] (1) respectively process ibuprofen arginine and codeine phosphate, hydroxypropyl ethyl cellulose, starch, micropowder silica gel of prescription quantity and cross 40 mesh sieves, mix homogeneously;
[0091] (2) Pack capsules.
Embodiment 3
[0092] Embodiment 3: Preparation of ibuprofen arginine codeine compound sustained-release tablet
[0093] Prescription (per 1000 tablets)
[0094] Sustained release tablet core:
[0095] Ibuprofen Arginine 400g
[0096] Lactose 30g
[0097] HPMC60g
[0098] (Hydroxypropylmethylcellulose)
[0099] Ethyl cellulose 20g
[0100] Magnesium stearate 8.2g
[0101] Immediate release layer:
[0102] Ibuprofen Arginine 500g
[0103] Codeine Phosphate 12.5g
[0104] Coloring layer:
[0105] Opadry 45g
[0106] Process:
[0107] (1) According to the above prescription, with 75% ethanol as a wetting agent, the sustained-release tablet core group is distributed with profen arginine, lactose, HPMC, ethyl cellulose and mixed evenly according to the prescription amount to make granules, add hard Magnesium fatty acid is used as a lubricant, and compressed into tablets to obtain sustained-release tablet cores;
[0108] (2) Ibuprofen arginine, codeine phosphate and 50% alcohol are mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com