Transgenic plant or plants with a naturally high water content overproducing at least two amino acids of the aspartate family
A technology of transgenic plants and aspartate kinase, which is applied in the fields of plant products, genetic engineering, plant genetic improvement, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The construction of the chimeric double gene construct of embodiment 1 expressing AK and DHPS
[0060] DNA isolation, subcloning, restriction analysis and DNA sequence analysis were performed using standard methods (Sambrook et al, 1989; Ausubel et al, 1994).
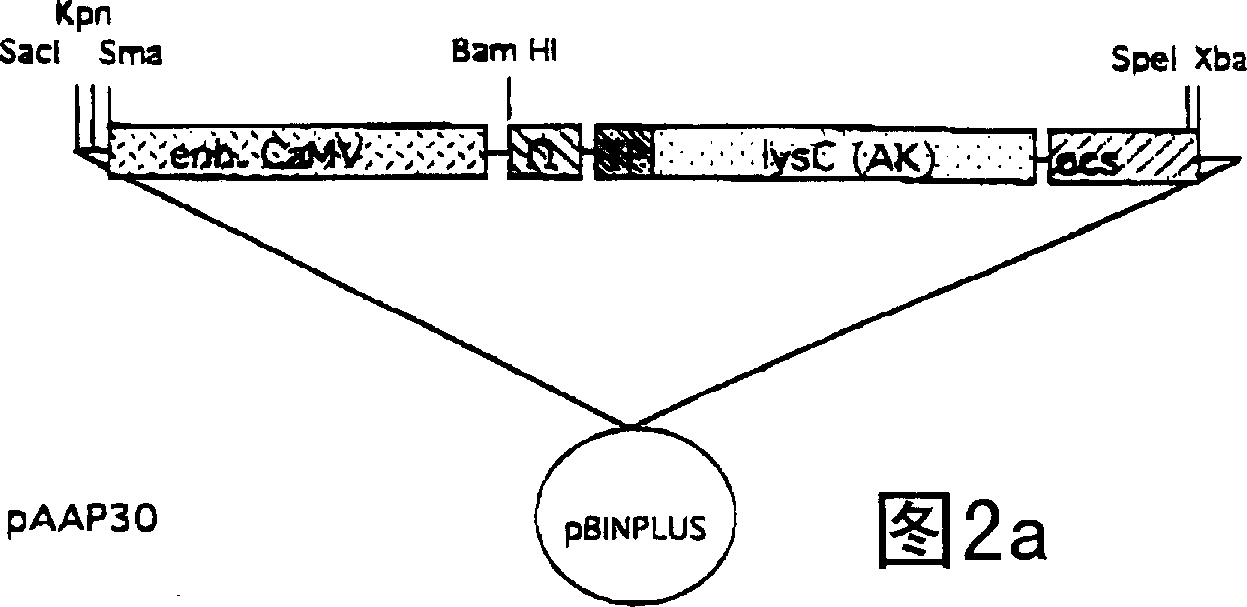
[0061] A chimeric gene containing the lysC gene was constructed by fusing the enhanced CaMV35S promoter to the chimeric lysC gene. This chimeric lysC construct contains a DNA segment of a mutated lysC allele fused at the 5' end to an omega DNA sequence derived from the envelope protein of tobacco mosaic virus (Gallie et al, 1989). Downstream of the lysC sequence, a termination signal derived from the octopine synthase gene of Agrobacterium tumefaciens was inserted (Greve et al, 1983). A DNA fragment (Fluhr et al, 1986) containing the coding sequence for the pea rbcS-3A transit peptide was inserted between the omega DNA and the lysC coding sequence. The chimeric lysC gene construct was cloned in pBluescript (Str...
Embodiment 2
[0064] Embodiment 2, chimeric gene is introduced in the potato
[0065] 2.1 Transformation of potato plants
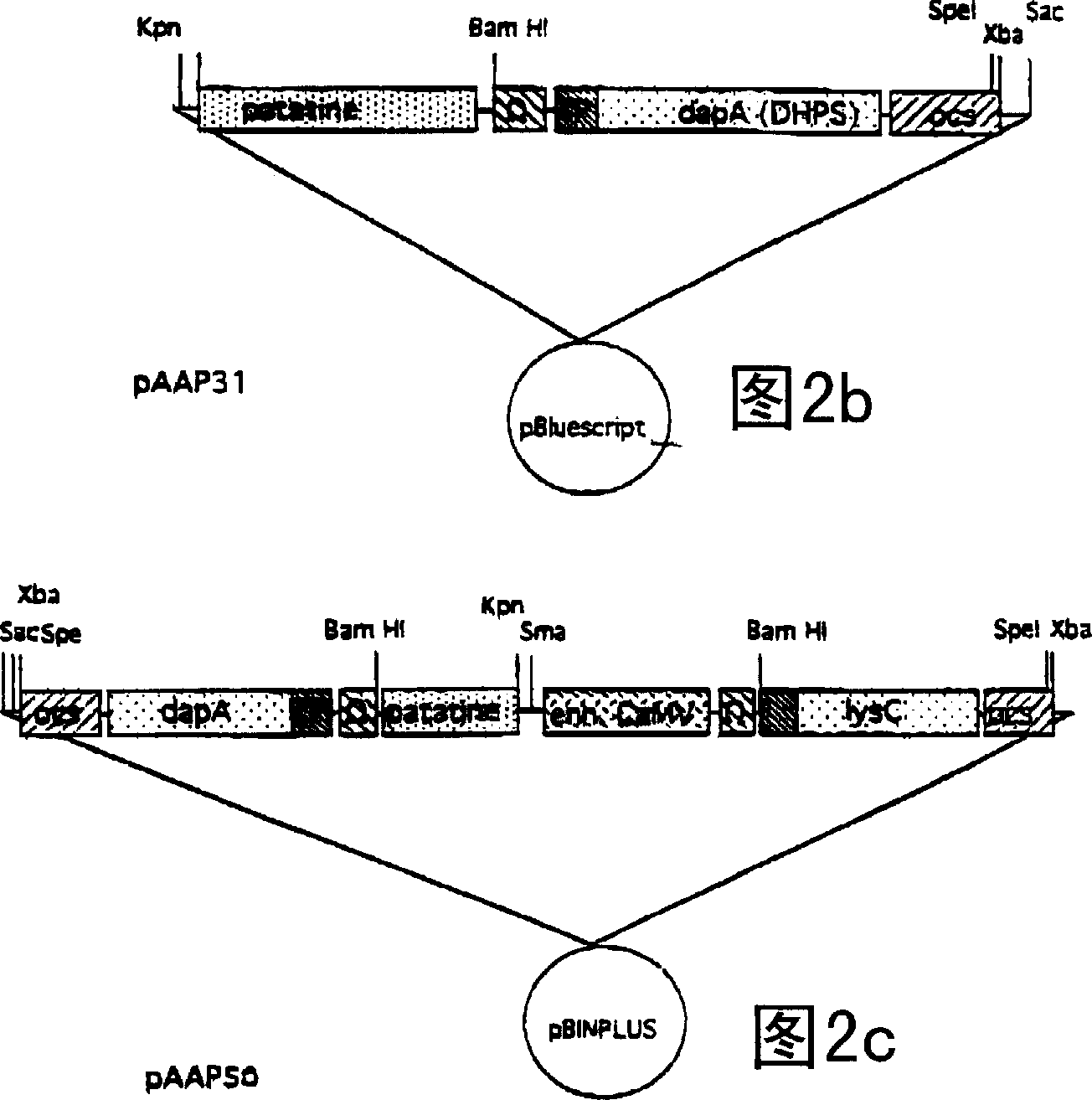
[0066]The binary vector pAAP50 was used for freeze-thaw transformation of Agrobacterium tumefaciens strain AGLO (Hofgen and Willmitzer 1988). The transformed AGLO was subsequently used for inoculation of diploid potato (Solanum tuberosum, var. Kardal) stem explants as described by Visser (1991). After regeneration of stems and roots on kanamycin-containing medium, plants were placed in soil and transferred to the greenhouse. Plants regenerated (on kanamycin free medium) from stem explants treated with Agrobacterium strain AGLO without the binary vector served as controls.
[0067] 2.2 Tuber formation in vitro
[0068] To induce tuber formation in vitro, excised nodes (about 4-5 cm long) of transformed potato plantlets were placed vertically on solid Murashige and Skoog (1962) medium supplemented with 10% (w / v) sucrose, 5 μM BAP middle. Cultures were maintained at ...
Embodiment 3
[0069] Example 3 Analysis of free lysine, threonine and methionine content in transgenic potato plants
[0070] Tissue (0.5-1.0 g) was homogenized with a mortar and pestle in 2 ml of 50 mM Pi buffer (pH 7.0) containing 1 mM dithiothreitol. Norleucine was added as an internal standard. The free amino acid was partially purified by extraction with 5 ml of a water:chloroform:methanol mixture (3:5:12). The aqueous phase was collected and extracted two more times. After concentration to 3 ml by lyophilization, 20 μl of the sample was used for 6-aminoquinolyl-N-hydroxysuccinimide carbamate (AQC) derivatization reaction. Derivatized amino acids were analyzed by DHPS on a reverse phase C18 column according to the manufacturer's instructions (WatersAccQ.Tag system)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com