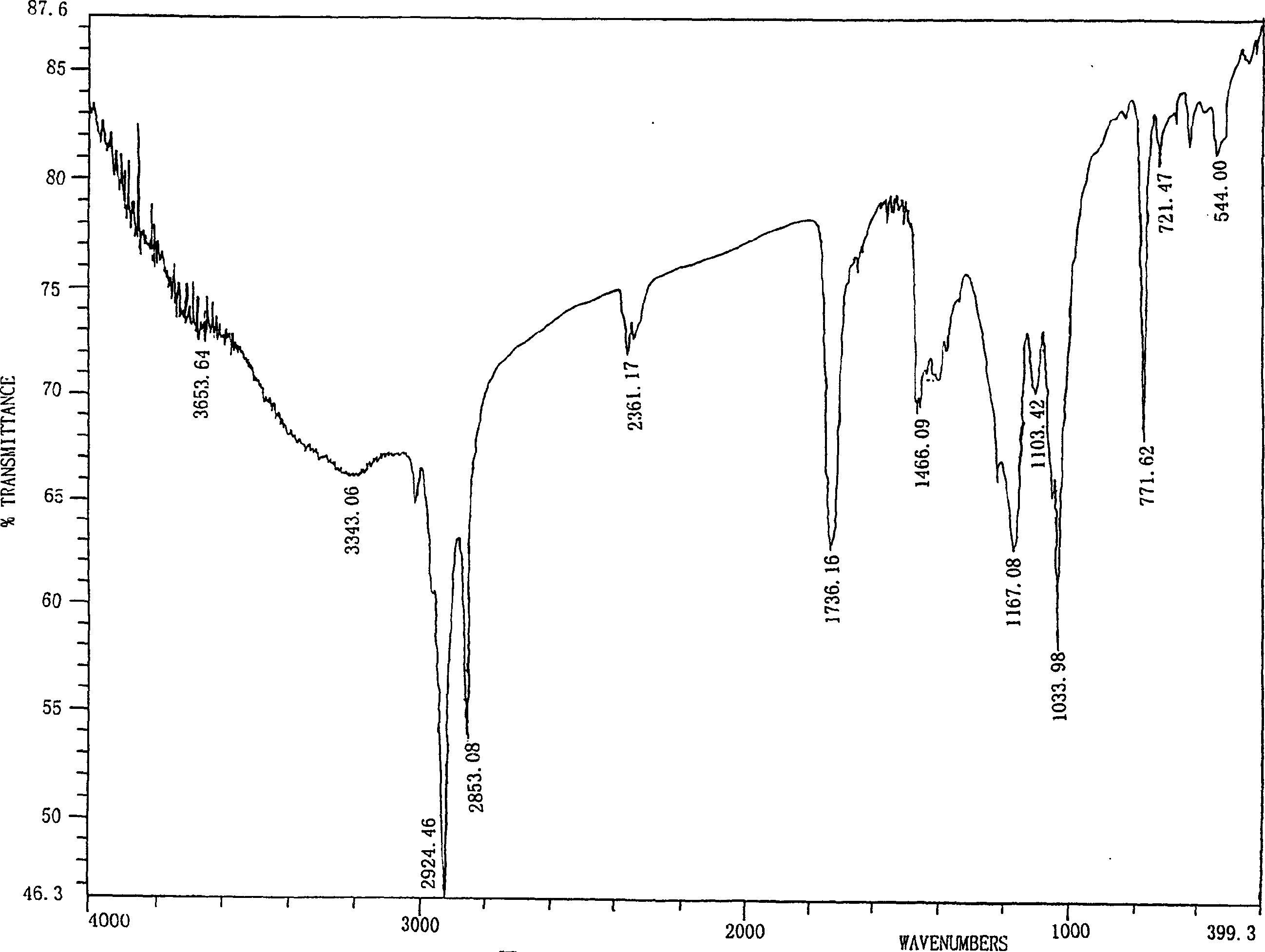
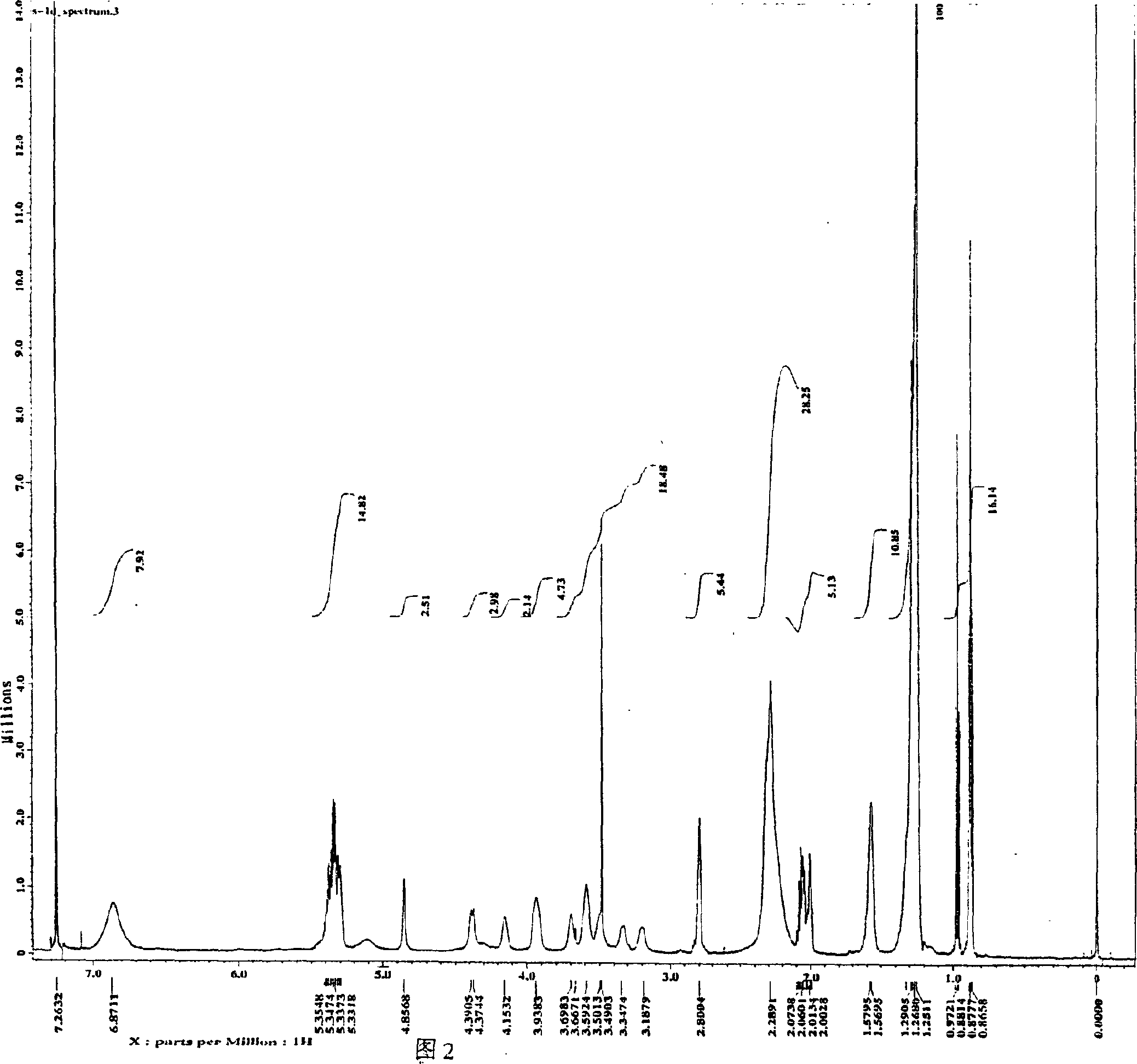
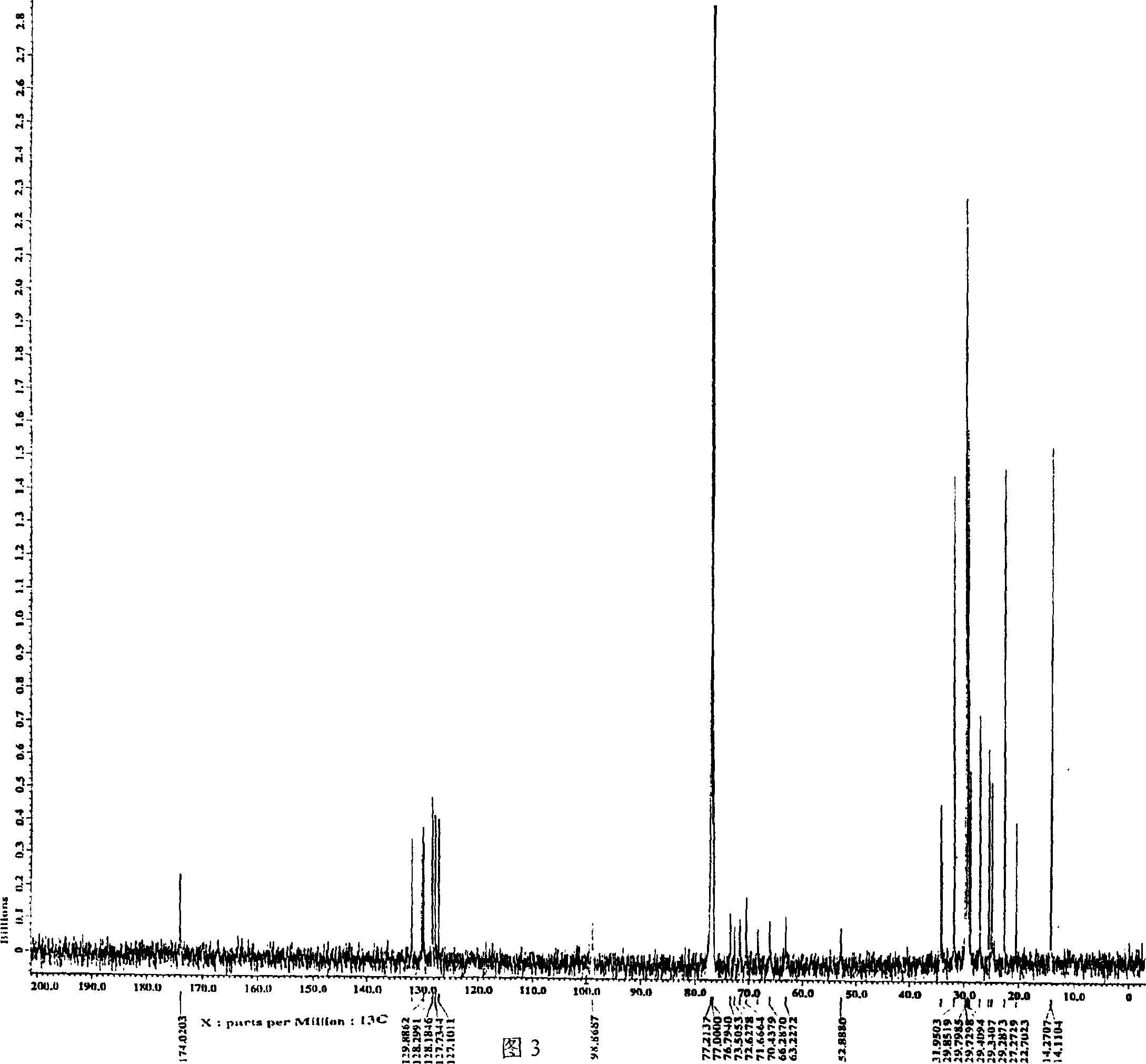
Preparation of 1,2-diacyl-3(6-deoxy-6-sulfo-alpha-D-glucopyranosyl)-sn-glycerin ester
A technology of sulfonic acid group and diacyl group, applied in 1 field, can solve problems such as unsuitable extraction and separation of preparation methods, limited sources of raw materials, etc., and achieve the effect of convenient research and development, rich raw materials and good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Extraction of total lipids from seaweed
[0027] Take 1 kg of fresh Ulva, freeze-dry and pulverize, add chloroform:methanol (1:1) 1L to soak for 24 hours, filter, filter residue and then soak for 10 hours in chloroform:methanol (1:1) 1L for a second time, filter and combine The filtrate was evaporated to dryness with a rotary evaporator under reduced pressure, then dissolved in 200 mL of chloroform-methanol (1: 1), and added 0.88% KCl solution, the final chloroform: methanol: the ratio of water was 1: 1: 0.9, and stood to separate layer, remove impurities, and evaporate the organic phase to dryness under reduced pressure to obtain 15.5 grams of total seaweed lipids;
[0028] 2. Pretreatment and packing of diethylaminoethyl cellulose
[0029] Weigh 15g of diethylaminoethyl cellulose, soak it with 1M HCl and 1M NaOH for 1.5 hours respectively, then wash it with methanol and chloroform which are twice the volume of diethylaminoethyl cellulose, and pump it with a Buchne...
Embodiment 2
[0042] 1. Extraction of total lipids from seaweed
[0043] Take 0.3 kg of dry turpentine algae, crush it, add 1 L of n-hexane-ethanol mixed solvent (1:1 volume ratio) to soak for 24 hours, filter, and soak the filter residue with 1 L of n-hexane-ethanol (1:1) mixed solvent for a second time After 10 hours, filter and combine the filtrates, evaporate to dryness with a rotary evaporator under reduced pressure, then dissolve in 100 mL of n-hexane: ethanol (1:1), and add 0.4% KCl solution, the final ratio of n-hexane: ethanol: water is 1 : 1: 0.6, standing for stratification, removing impurities, and evaporating the organic phase to dryness under reduced pressure to obtain 4.5 grams of total seaweed lipids;
[0044] 2. Pretreatment and packing of diethylaminoethyl cellulose
[0045] Weigh 15g of diethylaminoethyl dextran, soak it with 1M HCl and 1M NaOH for 3 hours, then wash with ethanol and n-hexane that are three times the volume of diethylaminoethyl dextran, and wash with a c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com