Method for renaturation of protein
A protein and denaturant technology, applied in the field of renatured proteins, can solve the problems of unsuitability for large-scale production, decreased activity recovery, and the volume cannot be too large, and achieves the effects of convenient large-scale application, simple operation, and reduced difficulty.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
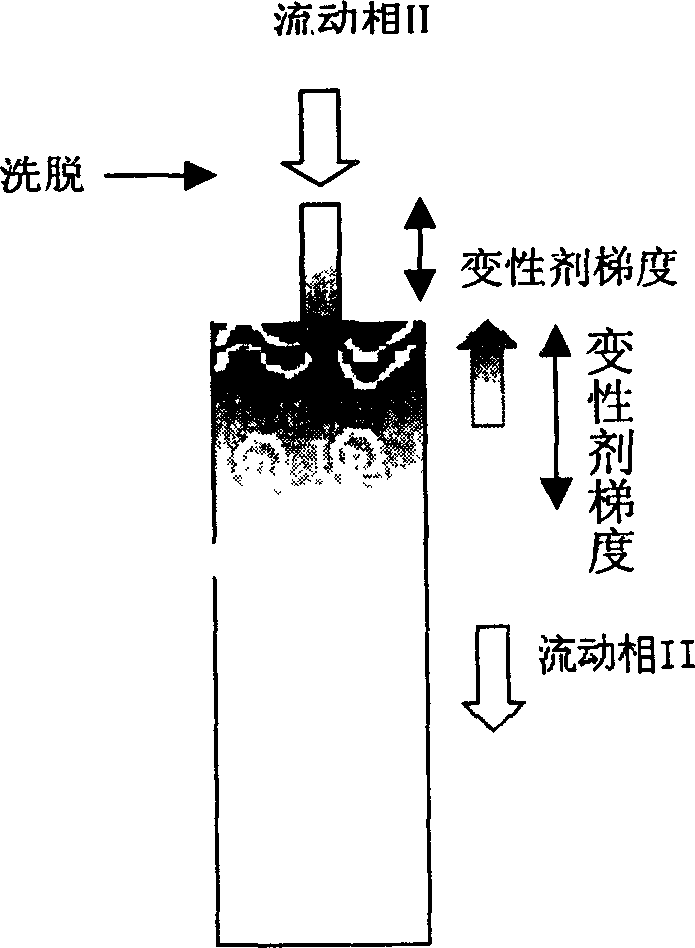
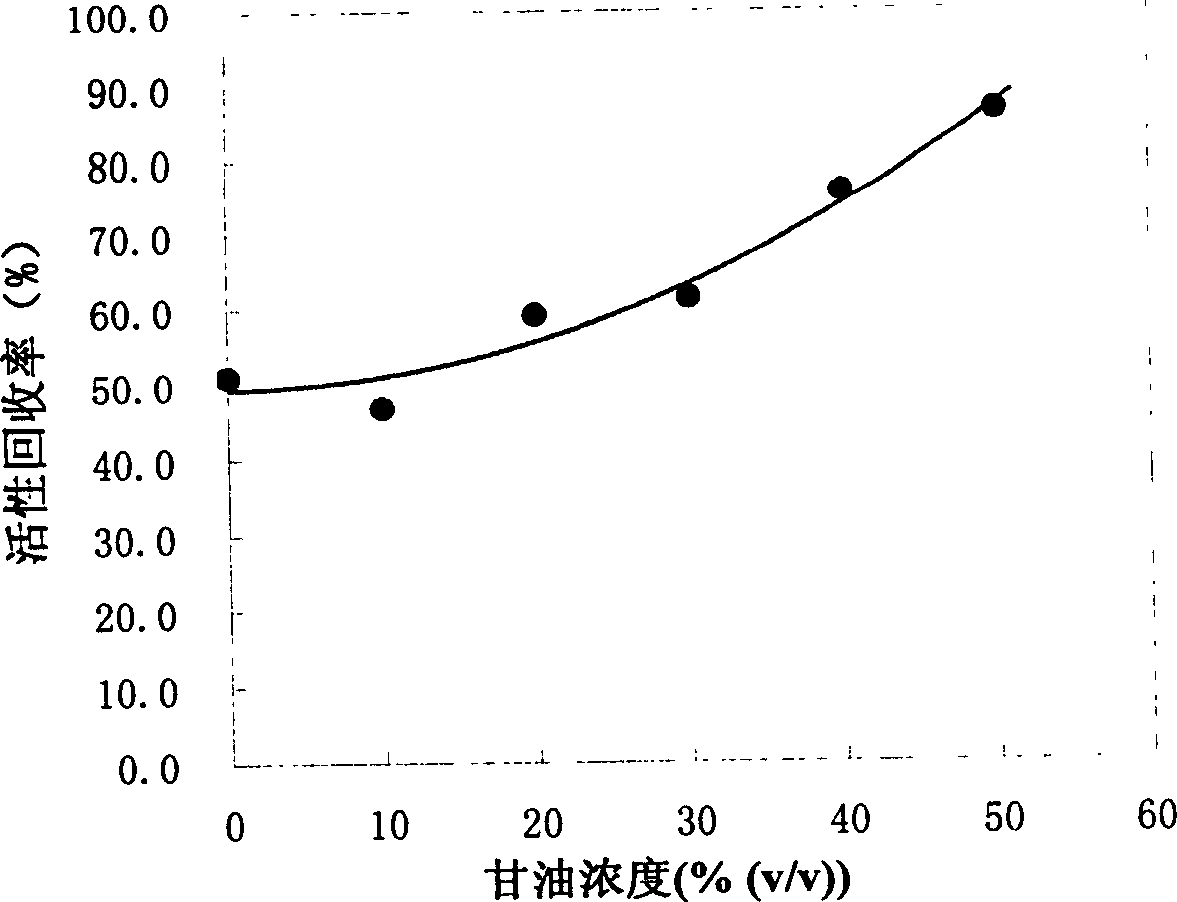
[0025] Renaturation of Denatured Lysozyme on Poros PE 50 Hydrophobic Chromatographic Column Eluted with Glycerol
[0026] Perfuse Poros PE 50 hydrophobic chromatography column (128mm×10mm I.D, 10mL) with mobile phase I (3.6M (NH 4 ) 2 SO 4 , 50mM Tris-HCl, 1mM EDTA, 3mM GSH, 0.3mM GSSG, pH8.5) equilibrated, run mobile phase I ~ denaturant A (8M urea, 50mM Tris-HCl, 1mM EDTA, 3mM GSH, 0.3mM GSSG, pH8.5) gradient 2ml, dissolving 10mg of lysozyme at a concentration of 50mg / ml with denaturant B (6M guanidine hydrochloride, 50mM Tris-HCl, 1mMEDTA, 1% 2-mercaptoethanol, pH8.5) and washing with mobile phase I to adsorb the denatured protein , and then change the eluent of mobile phase II and osmolyte (50% glycerol, 0.4M (NH 4 ) 2 SO 4 , 100mM Tris-HCL, 1mM EDTA, 0.1% 2-mercaptoethanol, pH8.5) to wash one column volume, and then run mobile phase II to denaturant C (8M urea, 50mM Tris-HCl, 1mM EDTA, 0.1% 2-mercaptoethanol, pH8.5) gradient 4ml and denaturant C ~ mobile phase II gr...
Embodiment 2
[0033] Refolding of recombinant human lysozyme inclusion body solution on Poros PE 50 hydrophobic chromatography column eluted with glycerol
[0034] A Poros PE 50 chromatography column (128mm×10mm I.D, 10mL) was used with mobile phase I (3.6M (NH 4 ) 2 SO 4 , 50mMTris-HCl, 1mM EDTA, 0.1% 2-mercaptoethanol, pH8.5) equilibrated, run the mobile phase I ~ denaturant A (8M urea, 50mMTris-HCl, 1mMEDTA, 3mMGSH, 0.3mM GSSG, pH8.5) gradient 2ml, containing denaturant B (6M guanidine hydrochloride, 50mM Tris-HCl, 1mM EDTA, 1% 2-mercaptoethanol, pH8.5) human lysozyme solution 2mg, wash with mobile phase I, let the denatured protein adsorb, and then change the flow Phase II (50% glycerol, 0.4M (NH 4 ) 2 SO 4 , 100mM Tris-HCL, 1mM EDTA, 0.1% 2-mercaptoethanol, pH8.5) to wash one column volume, and then run mobile phase II to denaturant C (8M urea, 50mM Tris-HCl, 1mM EDTA, 0.1% 2-mercaptoethanol , pH8.5) gradient 5ml and denaturant C ~ mobile phase II gradient 5ml, continue to elute ...
Embodiment 3
[0038] Refolding of Recombinant Human α-2b Interferon on Phenyl Sepharose FF Hydrophobic Chromatography Column Eluted with Mannitol
[0039] Equilibrate the Phenyl Sepharose FF chromatography column (25mm×16mm I.D, 5mL) with mobile phase I (0.1N acetic acid), and denature it with 6M guanidine hydrochloride, 50mM Tris-HCl, 1mMEDTA, 1% 2-mercaptoethanol, pH8.5 The reduced recombinant α-2b interferon inclusion body was washed with mobile phase I to absorb the denatured protein, and then changed to mobile phase II (20% mannitol, 0.2M NaCl, 20mM Na 2 HPO 4 , 0.01% 2-mercaptoethanol) to wash a column volume, and then run mobile phase II ~ denaturant C (8M urea, 10mM Na 2 HPO 4 , 0.01% 2-mercaptoethanol) gradient 5ml and denaturant C ~ mobile phase II gradient 5ml, continue to elute with mobile phase II, the flow rate is 2ml / min, and the specific activity of α-2b interferon eluted is 1.5× 10 8 IU / mg, the total activity yield is up to 80%.
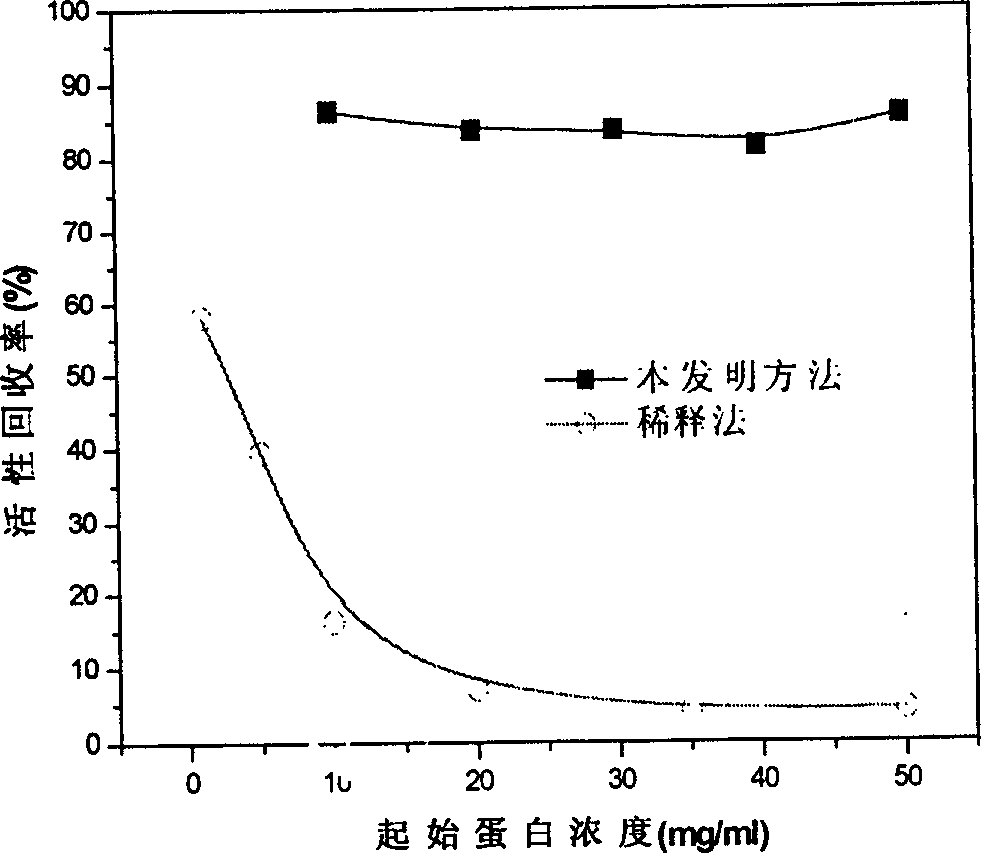
[0040] It shows that the present inven...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com