Method for pre-column derivatization for sulfonyl isocyanic ester of hydroxyl compound and sulfhydryl commpound
A technology of sulfonyl isocyanate and pre-column derivatization, which is applied in the field of analytical chemistry, can solve the problems of no pre-column derivatization, etc., and achieve the effect of high reaction time and less side reaction products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
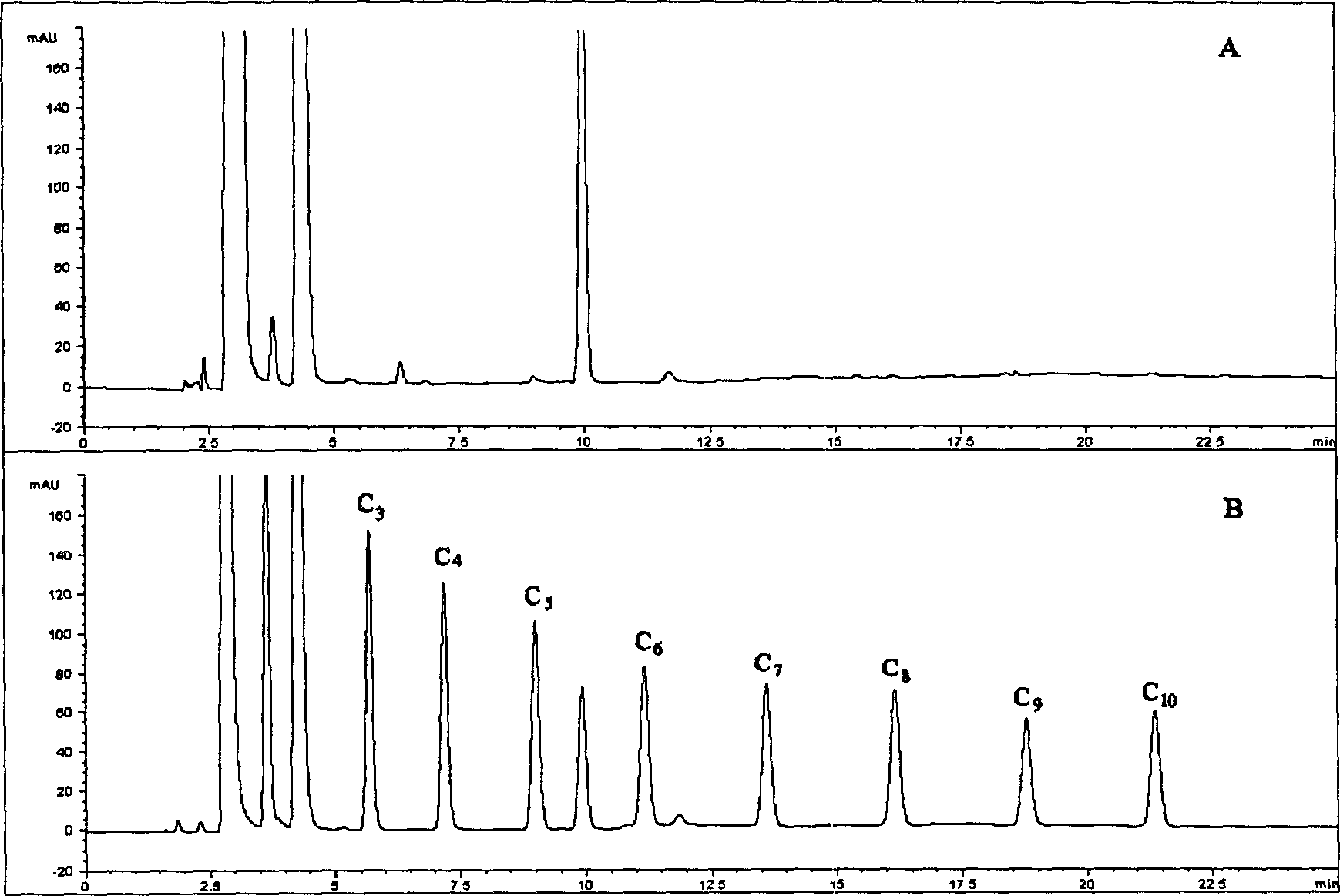
[0019] Instruments and Conditions
[0020] American Agilent 1100 high performance liquid chromatography system, including low pressure quaternary gradient system, online degassing, column temperature control box, variable wavelength detector, ChemStation for LC 3D workstation and 20μl manual sampling system; chromatographic column: 1 50× 4.6mm ID, 5μm Diamonsil TM C 18 (DIKMA); mobile phase A: weigh 1.79 grams of NaH 2 PO 4 2H 2 Dissolve O in 250ml of pure water, add 250ml of acetonitrile, and adjust the pH value to 2.5 with phosphoric acid; mobile phase B: weigh 1.79g of NaH 2 PO 4 2H 2 Dissolve O in 100ml of pure water, add 400ml of acetonitrile, and adjust the pH value to 2.5 with phosphoric acid; gradient program:
[0021] T(min) A(%) B(%)
[0022] 0 100 0
[0023] 20 0 100
[0024] 25 0 100
[0025] Flow rate: 1.0ml / min; Column temperature: 25°C; Detection wavelength: 227nm
[0026] Experimental procedure
[0027] Ta...
Embodiment 2
[0029] Instruments and Conditions
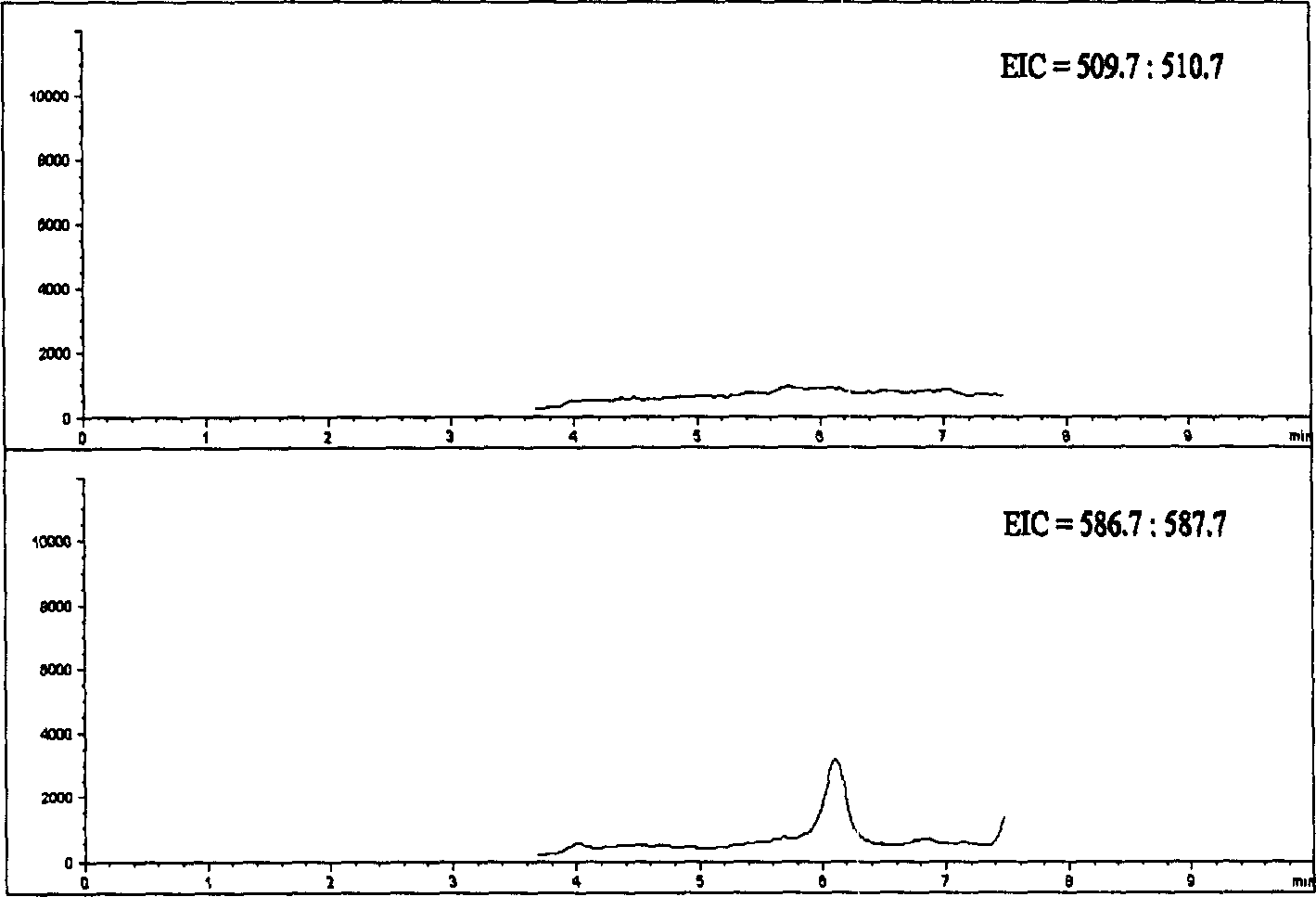
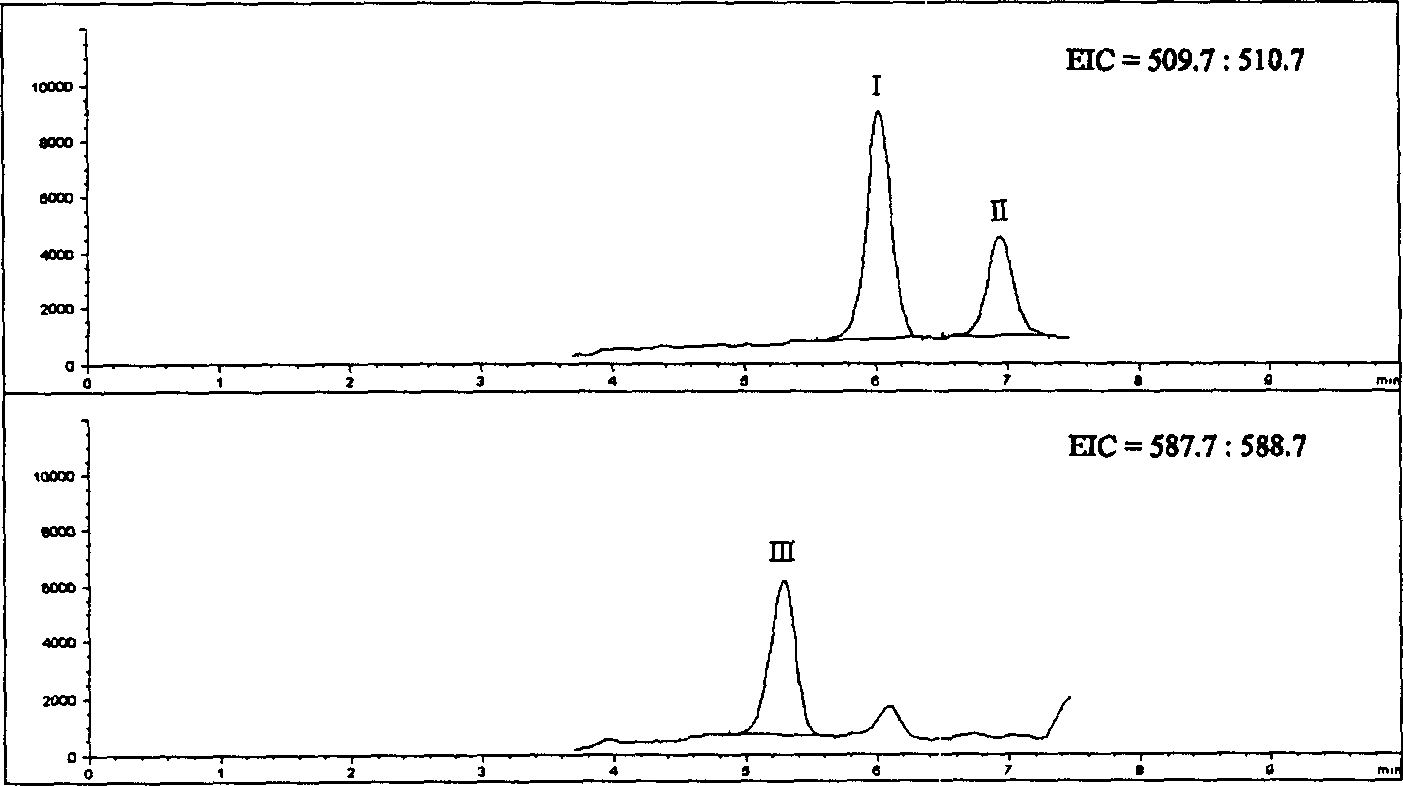
[0030] American Agilent 1100 HPLC-MSD high-performance liquid chromatography-mass spectrometry system, the system includes online degassing, binary high-pressure gradient system, column temperature control box, automatic sampler, diode array detector, electrospray interface (ion source), Quadrupole mass analysis system and ChemStation for LC-MS 3D workstation.
[0031] Chromatographic conditions Column: 200×4.6mm ID, 5μm Diamonsil TM C 18 (DIKMA); mobile phase A: 0.2% ammonium acetate solution (g / ml); mobile phase B: methanol; gradient elution program
[0032] T(min) A(%) B(%)
[0033] 0 22 78
[0034] 10 0 100
[0035] Flow rate: 0.8ml / min; Column temperature: 25°C
[0036] Mass spectrometry conditions ESI negative ion ionization; fragmentation voltage: 130V; spray pressure: 40psi; nitrogen flow rate: 10L / min; drying air temperature: 350°C; capillary voltage: 4000V; 1 =511.0 (Org 4094 and Org 30126), M 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com